+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7oks | ||||||
|---|---|---|---|---|---|---|---|
| Title | X-ray structure of soluble EPCR in P212121 space group | ||||||
 Components Components | Endothelial protein C receptor | ||||||
 Keywords Keywords | LIPID BINDING PROTEIN / MHC class I-like / PHOSPHOLIPID / ANTICOAGULANT / ENDOTHELIAL CELL MEMBRANE RECEPTOR. | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of coagulation / Common Pathway of Fibrin Clot Formation / Cell surface interactions at the vascular wall / blood coagulation / signaling receptor activity / focal adhesion / centrosome / perinuclear region of cytoplasm / cell surface / extracellular space ...negative regulation of coagulation / Common Pathway of Fibrin Clot Formation / Cell surface interactions at the vascular wall / blood coagulation / signaling receptor activity / focal adhesion / centrosome / perinuclear region of cytoplasm / cell surface / extracellular space / extracellular exosome / extracellular region / plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.95 Å MOLECULAR REPLACEMENT / Resolution: 1.95 Å | ||||||
 Authors Authors | Erausquin, E. / Dichiara, M.G. / Lopez-Sagaseta, J. | ||||||
| Funding support |  Spain, 1items Spain, 1items
| ||||||
 Citation Citation |  Journal: Sci Rep / Year: 2022 Journal: Sci Rep / Year: 2022Title: Identification of a broad lipid repertoire associated to the endothelial cell protein C receptor (EPCR). Authors: Erausquin, E. / Moran-Garrido, M. / Saiz, J. / Barbas, C. / Dichiara-Rodriguez, G. / Urdiciain, A. / Lopez-Sagaseta, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7oks.cif.gz 7oks.cif.gz | 371.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7oks.ent.gz pdb7oks.ent.gz | 254.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7oks.json.gz 7oks.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7oks_validation.pdf.gz 7oks_validation.pdf.gz | 5.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7oks_full_validation.pdf.gz 7oks_full_validation.pdf.gz | 5.1 MB | Display | |
| Data in XML |  7oks_validation.xml.gz 7oks_validation.xml.gz | 32.7 KB | Display | |
| Data in CIF |  7oks_validation.cif.gz 7oks_validation.cif.gz | 44.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ok/7oks https://data.pdbj.org/pub/pdb/validation_reports/ok/7oks ftp://data.pdbj.org/pub/pdb/validation_reports/ok/7oks ftp://data.pdbj.org/pub/pdb/validation_reports/ok/7oks | HTTPS FTP |
-Related structure data
| Related structure data |  7oktC  7okuC  7okvC  1lqvS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 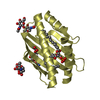
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
|
 Movie
Movie Controller
Controller



 PDBj
PDBj



