[English] 日本語
 Yorodumi
Yorodumi- PDB-7m0a: Incomplete in crystallo incorporation by DNA Polymerase Lambda bo... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7m0a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Incomplete in crystallo incorporation by DNA Polymerase Lambda bound to blunt-ended DSB substrate and incoming dTTP | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | TRANSFERASE / Nonhomologous end-joining / Base Excision Repair | |||||||||
| Function / homology |  Function and homology information Function and homology informationDNA biosynthetic process / Lyases; Carbon-oxygen lyases; Other carbon-oxygen lyases / 5'-deoxyribose-5-phosphate lyase activity / somatic hypermutation of immunoglobulin genes / base-excision repair, gap-filling / nucleotide-excision repair / Nonhomologous End-Joining (NHEJ) / double-strand break repair via homologous recombination / double-strand break repair via nonhomologous end joining / site of double-strand break ...DNA biosynthetic process / Lyases; Carbon-oxygen lyases; Other carbon-oxygen lyases / 5'-deoxyribose-5-phosphate lyase activity / somatic hypermutation of immunoglobulin genes / base-excision repair, gap-filling / nucleotide-excision repair / Nonhomologous End-Joining (NHEJ) / double-strand break repair via homologous recombination / double-strand break repair via nonhomologous end joining / site of double-strand break / DNA-directed DNA polymerase / DNA-directed DNA polymerase activity / DNA replication / DNA binding / nucleoplasm / metal ion binding / nucleus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)synthetic construct (others) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.83 Å MOLECULAR REPLACEMENT / Resolution: 1.83 Å | |||||||||
 Authors Authors | Kaminski, A.M. / Bebenek, K. / Pedersen, L.C. / Kunkel, T.A. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2022 Journal: Nat Commun / Year: 2022Title: Analysis of diverse double-strand break synapsis with Pol lambda reveals basis for unique substrate specificity in nonhomologous end-joining. Authors: Kaminski, A.M. / Chiruvella, K.K. / Ramsden, D.A. / Bebenek, K. / Kunkel, T.A. / Pedersen, L.C. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7m0a.cif.gz 7m0a.cif.gz | 209.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7m0a.ent.gz pdb7m0a.ent.gz | 140.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7m0a.json.gz 7m0a.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/m0/7m0a https://data.pdbj.org/pub/pdb/validation_reports/m0/7m0a ftp://data.pdbj.org/pub/pdb/validation_reports/m0/7m0a ftp://data.pdbj.org/pub/pdb/validation_reports/m0/7m0a | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 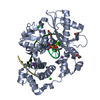 7m07C  7m09C  7m0bC  7m0dC  7m0eC  2pfoS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 38550.961 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: POLL / Plasmid: pGEXM / Production host: Homo sapiens (human) / Gene: POLL / Plasmid: pGEXM / Production host:  References: UniProt: Q9UGP5, DNA-directed DNA polymerase, Lyases; Carbon-oxygen lyases; Other carbon-oxygen lyases |
|---|
-DNA chain , 4 types, 4 molecules TPDU
| #2: DNA chain | Mass: 1505.025 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
|---|---|
| #3: DNA chain | Mass: 2113.410 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
| #4: DNA chain | Mass: 1191.818 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
| #5: DNA chain | Mass: 1809.217 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) synthetic construct (others) |
-Non-polymers , 8 types, 412 molecules 
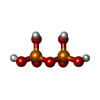













| #6: Chemical | ChemComp-TTP / | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #7: Chemical | ChemComp-PPV / | ||||||||||
| #8: Chemical | | #9: Chemical | ChemComp-CA / | #10: Chemical | #11: Chemical | #12: Chemical | ChemComp-GOL / | #13: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3 Å3/Da / Density % sol: 59.1 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 80mM Na cacodylate pH 6.5, 0.16M calcium acetate, 14.4% (w/v) PEG 8000, 20% (v/v) glycerol |
-Data collection
| Diffraction | Mean temperature: 93 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-ID / Wavelength: 1 Å / Beamline: 22-ID / Wavelength: 1 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Sep 25, 2020 |
| Radiation | Monochromator: DOUBLE CYRSTAL SI(III) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.83→50 Å / Num. obs: 41774 / % possible obs: 99 % / Redundancy: 6.7 % / Biso Wilson estimate: 23.35 Å2 / CC1/2: 0.997 / CC star: 0.999 / Rsym value: 0.072 / Χ2: 0.457 / Net I/σ(I): 18.3 |
| Reflection shell | Resolution: 1.83→1.86 Å / Redundancy: 6.6 % / Mean I/σ(I) obs: 1.62 / Num. unique obs: 2071 / CC1/2: 0.75 / CC star: 0.926 / Rsym value: 0.703 / Χ2: 0.334 / % possible all: 98.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2PFO Resolution: 1.83→43.71 Å / SU ML: 0.2043 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 18.1618 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 26.5 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.83→43.71 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Refine-ID: X-RAY DIFFRACTION
|
 Movie
Movie Controller
Controller











 PDBj
PDBj











