+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7jgu | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of FN3tt mut | ||||||
 Components Components | Fibronectin type-III domain-containing protein | ||||||
 Keywords Keywords | STRUCTURAL PROTEIN / FN3-like domain / Hyperthermal stability / Surface Salt Bridges | ||||||
| Function / homology |  Function and homology information Function and homology information: / IPT/TIG domain / ig-like, plexins, transcription factors / IPT domain / Fibronectin type III domain / Fibronectin type 3 domain / Fibronectin type-III domain profile. / Fibronectin type III / Fibronectin type III superfamily / Immunoglobulin E-set / Immunoglobulin-like fold Similarity search - Domain/homology | ||||||
| Biological species |  Caldanaerobacter subterraneus subsp. tengcongensis (bacteria) Caldanaerobacter subterraneus subsp. tengcongensis (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.2 Å MOLECULAR REPLACEMENT / Resolution: 1.2 Å | ||||||
 Authors Authors | Luo, J. / Boucher, L.E. | ||||||
 Citation Citation |  Journal: Proteins / Year: 2022 Journal: Proteins / Year: 2022Title: Surface salt bridges contribute to the extreme thermal stability of an FN3-like domain from a thermophilic bacterium. Authors: Boucher, L. / Somani, S. / Negron, C. / Ma, W. / Jacobs, S. / Chan, W. / Malia, T. / Obmolova, G. / Teplyakov, A. / Gilliland, G.L. / Luo, J. | ||||||
| History |
|
- Structure visualization
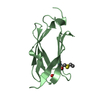
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7jgu.cif.gz 7jgu.cif.gz | 73.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7jgu.ent.gz pdb7jgu.ent.gz | 53.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7jgu.json.gz 7jgu.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jg/7jgu https://data.pdbj.org/pub/pdb/validation_reports/jg/7jgu ftp://data.pdbj.org/pub/pdb/validation_reports/jg/7jgu ftp://data.pdbj.org/pub/pdb/validation_reports/jg/7jgu | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7jgtSC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
| ||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 11175.329 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Caldanaerobacter subterraneus subsp. tengcongensis (strain DSM 15242 / JCM 11007 / NBRC 100824 / MB4) (bacteria) Caldanaerobacter subterraneus subsp. tengcongensis (strain DSM 15242 / JCM 11007 / NBRC 100824 / MB4) (bacteria)Strain: DSM 15242 / JCM 11007 / NBRC 100824 / MB4 / Gene: TTE0165 / Production host:  |
|---|---|
| #2: Chemical | ChemComp-1PE / |
| #3: Water | ChemComp-HOH / |
| Has ligand of interest | N |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.49 Å3/Da / Density % sol: 50.51 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: evaporation / pH: 7.5 / Details: 25% PEG 3350, 0.2M sodium di-hydrogen phosphate |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 17-ID / Wavelength: 1 Å / Beamline: 17-ID / Wavelength: 1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Oct 11, 2011 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.2→27.36 Å / Num. obs: 34160 / % possible obs: 99 % / Redundancy: 3.184 % / Biso Wilson estimate: 19.682 Å2 / CC1/2: 0.998 / Rmerge(I) obs: 0.05 / Rrim(I) all: 0.06 / Χ2: 0.93 / Net I/σ(I): 12.39 / Num. measured all: 108762 / Scaling rejects: 9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 7JGT Resolution: 1.2→27.36 Å / SU ML: 0.15 / Cross valid method: THROUGHOUT / σ(F): 1.37 / Phase error: 19.65 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 135.71 Å2 / Biso mean: 24.7027 Å2 / Biso min: 8.07 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.2→27.36 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 12
|
 Movie
Movie Controller
Controller












 PDBj
PDBj






