[English] 日本語
 Yorodumi
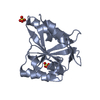
Yorodumi- PDB-7ber: SFX structure of the MyD88 TIR domain higher-order assembly (solv... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7ber | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SFX structure of the MyD88 TIR domain higher-order assembly (solved, rebuilt and refined using an identical protocol to the MicroED structure of the MyD88 TIR domain higher-order assembly) | |||||||||
 Components Components | Myeloid differentiation primary response protein MyD88 | |||||||||
 Keywords Keywords | PROTEIN BINDING / SFX / serial femtosecond crystallography / MyD88 / TIR domain / higher-order assembly | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of chemokine (C-X-C motif) ligand 1 production / MyD88 deficiency (TLR5) / TIR domain binding / Toll binding / toll-like receptor 5 signaling pathway / induced systemic resistance / ATP-dependent histone chaperone activity / regulation of chemokine (C-X-C motif) ligand 2 production / neutrophil-mediated killing of bacterium / leukocyte activation involved in inflammatory response ...regulation of chemokine (C-X-C motif) ligand 1 production / MyD88 deficiency (TLR5) / TIR domain binding / Toll binding / toll-like receptor 5 signaling pathway / induced systemic resistance / ATP-dependent histone chaperone activity / regulation of chemokine (C-X-C motif) ligand 2 production / neutrophil-mediated killing of bacterium / leukocyte activation involved in inflammatory response / response to molecule of fungal origin / positive regulation of lymphocyte proliferation / toll-like receptor 8 signaling pathway / response to peptidoglycan / positive regulation of interleukin-23 production / establishment of endothelial intestinal barrier / regulation of neutrophil migration / IRAK4 deficiency (TLR5) / MyD88 dependent cascade initiated on endosome / Toll-like receptor binding / cellular response to oxidised low-density lipoprotein particle stimulus / DEx/H-box helicases activate type I IFN and inflammatory cytokines production / TRAF6 mediated induction of NFkB and MAP kinases upon TLR7/8 or 9 activation / MyD88 cascade initiated on plasma membrane / toll-like receptor TLR6:TLR2 signaling pathway / Toll signaling pathway / interleukin-33-mediated signaling pathway / neutrophil activation involved in immune response / microglia differentiation / RIP-mediated NFkB activation via ZBP1 / MyD88 deficiency (TLR2/4) / interleukin-1 receptor binding / positive regulation of cytokine production involved in inflammatory response / death receptor binding / response to amine / interleukin-1-mediated signaling pathway / IRAK4 deficiency (TLR2/4) / extrinsic component of cytoplasmic side of plasma membrane / MyD88:MAL(TIRAP) cascade initiated on plasma membrane / 3'-UTR-mediated mRNA stabilization / MyD88-dependent toll-like receptor signaling pathway / positive regulation of macrophage cytokine production / extrinsic component of plasma membrane / toll-like receptor 4 signaling pathway / skin development / type I interferon-mediated signaling pathway / defense response to protozoan / positive regulation of interleukin-17 production / positive regulation of NLRP3 inflammasome complex assembly / immunoglobulin mediated immune response / response to amino acid / phagocytosis / positive regulation of chemokine production / positive regulation of type I interferon production / JNK cascade / positive regulation of smooth muscle cell proliferation / signaling adaptor activity / response to interleukin-1 / p75NTR recruits signalling complexes / TRAF6 mediated IRF7 activation in TLR7/8 or 9 signaling / lipopolysaccharide-mediated signaling pathway / positive regulation of interleukin-1 beta production / positive regulation of interleukin-8 production / cellular response to mechanical stimulus / : / positive regulation of JNK cascade / positive regulation of interleukin-6 production / Interleukin-1 signaling / positive regulation of tumor necrosis factor production / PIP3 activates AKT signaling / ER-Phagosome pathway / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / regulation of inflammatory response / cellular response to lipopolysaccharide / gene expression / defense response to virus / response to ethanol / molecular adaptor activity / cell surface receptor signaling pathway / positive regulation of canonical NF-kappaB signal transduction / endosome membrane / defense response to bacterium / defense response to Gram-positive bacterium / innate immune response / apoptotic process / positive regulation of gene expression / cell surface / signal transduction / positive regulation of transcription by RNA polymerase II / protein-containing complex / identical protein binding / nucleus / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FREE ELECTRON LASER / FREE ELECTRON LASER /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | |||||||||
 Authors Authors | Clabbers, M.T.B. / Holmes, S. / Muusse, T.W. / Vajjhala, P. / Thygesen, S.J. / Malde, A.K. / Hunter, D.J.B. / Croll, T.I. / Nanson, J.D. / Rahaman, M.H. ...Clabbers, M.T.B. / Holmes, S. / Muusse, T.W. / Vajjhala, P. / Thygesen, S.J. / Malde, A.K. / Hunter, D.J.B. / Croll, T.I. / Nanson, J.D. / Rahaman, M.H. / Aquila, A. / Hunter, M.S. / Liang, M. / Yoon, C.H. / Zhao, J. / Zatsepin, N.A. / Abbey, B. / Sierecki, E. / Gambin, Y. / Darmanin, C. / Kobe, B. / Xu, H. / Ve, T. | |||||||||
| Funding support |  Sweden, Sweden,  Australia, 2items Australia, 2items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: MyD88 TIR domain higher-order assembly interactions revealed by microcrystal electron diffraction and serial femtosecond crystallography. Authors: Max T B Clabbers / Susannah Holmes / Timothy W Muusse / Parimala R Vajjhala / Sara J Thygesen / Alpeshkumar K Malde / Dominic J B Hunter / Tristan I Croll / Leonie Flueckiger / Jeffrey D ...Authors: Max T B Clabbers / Susannah Holmes / Timothy W Muusse / Parimala R Vajjhala / Sara J Thygesen / Alpeshkumar K Malde / Dominic J B Hunter / Tristan I Croll / Leonie Flueckiger / Jeffrey D Nanson / Md Habibur Rahaman / Andrew Aquila / Mark S Hunter / Mengning Liang / Chun Hong Yoon / Jingjing Zhao / Nadia A Zatsepin / Brian Abbey / Emma Sierecki / Yann Gambin / Katryn J Stacey / Connie Darmanin / Bostjan Kobe / Hongyi Xu / Thomas Ve /     Abstract: MyD88 and MAL are Toll-like receptor (TLR) adaptors that signal to induce pro-inflammatory cytokine production. We previously observed that the TIR domain of MAL (MAL) forms filaments in vitro and ...MyD88 and MAL are Toll-like receptor (TLR) adaptors that signal to induce pro-inflammatory cytokine production. We previously observed that the TIR domain of MAL (MAL) forms filaments in vitro and induces formation of crystalline higher-order assemblies of the MyD88 TIR domain (MyD88). These crystals are too small for conventional X-ray crystallography, but are ideally suited to structure determination by microcrystal electron diffraction (MicroED) and serial femtosecond crystallography (SFX). Here, we present MicroED and SFX structures of the MyD88 assembly, which reveal a two-stranded higher-order assembly arrangement of TIR domains analogous to that seen previously for MAL. We demonstrate via mutagenesis that the MyD88 assembly interfaces are critical for TLR4 signaling in vivo, and we show that MAL promotes unidirectional assembly of MyD88. Collectively, our studies provide structural and mechanistic insight into TLR signal transduction and allow a direct comparison of the MicroED and SFX techniques. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7ber.cif.gz 7ber.cif.gz | 49.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7ber.ent.gz pdb7ber.ent.gz | 27.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7ber.json.gz 7ber.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/be/7ber https://data.pdbj.org/pub/pdb/validation_reports/be/7ber ftp://data.pdbj.org/pub/pdb/validation_reports/be/7ber ftp://data.pdbj.org/pub/pdb/validation_reports/be/7ber | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7beqC  7l6wC  4w8gS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | |
| Experimental dataset #1 | Data reference:  10.11577/1767965 / Data set type: diffraction image data 10.11577/1767965 / Data set type: diffraction image data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 17833.854 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: MYD88 / Production host: Homo sapiens (human) / Gene: MYD88 / Production host:  |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.31 Å3/Da / Density % sol: 46.65 % |
|---|---|
| Crystal grow | Temperature: 310 K / Method: batch mode Details: MAL TIR (0.5-3 mM) incubated with MyD88 TIR (60-100 mM) in 10 mM HEPES pH 7.5-8, 150 mM NaCl at 310K |
-Data collection
| Diffraction | Mean temperature: 300 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  FREE ELECTRON LASER / Site: FREE ELECTRON LASER / Site:  SLAC LCLS SLAC LCLS  / Beamline: CXI / Wavelength: 1.3 Å / Beamline: CXI / Wavelength: 1.3 Å |
| Detector | Type: CS-PAD CXI-1 / Detector: PIXEL / Date: Dec 17, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.3 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→30.94 Å / Num. obs: 7118 / % possible obs: 95.8 % / Redundancy: 1 % / Biso Wilson estimate: 32.47 Å2 / CC1/2: 0.9 / Net I/σ(I): 2.6 |
| Reflection shell | Resolution: 2.3→2.38 Å / Redundancy: 1 % / Mean I/σ(I) obs: 1.1 / Num. unique obs: 560 / CC1/2: 0.36 / % possible all: 78.1 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4W8G Resolution: 2.3→30.93 Å / SU ML: 0.2669 / Cross valid method: THROUGHOUT / σ(F): 1.91 / Phase error: 39.873 / Stereochemistry target values: CDL v1.2
| ||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 45.6 Å2 | ||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→30.93 Å
| ||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj










