+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7au6 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cytochrome c oxidase structure in O-state | |||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components | (Cytochrome c oxidase subunit ...) x 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / Terminal oxidase Cytochrome c oxidase aa3 oxidase | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationaerobic electron transport chain / respiratory chain complex IV / cytochrome-c oxidase / oxidative phosphorylation / cytochrome-c oxidase activity / electron transport coupled proton transport / ATP synthesis coupled electron transport / respiratory electron transport chain / copper ion binding / heme binding ...aerobic electron transport chain / respiratory chain complex IV / cytochrome-c oxidase / oxidative phosphorylation / cytochrome-c oxidase activity / electron transport coupled proton transport / ATP synthesis coupled electron transport / respiratory electron transport chain / copper ion binding / heme binding / metal ion binding / plasma membrane Similarity search - Function | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  Paracoccus denitrificans (bacteria) Paracoccus denitrificans (bacteria) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.4 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Kolbe, F. / Safarian, S. / Michel, H. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  Germany, 1items Germany, 1items
| |||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Cryo-EM structures of intermediates suggest an alternative catalytic reaction cycle for cytochrome c oxidase. Authors: F Kolbe / S Safarian / Ż Piórek / S Welsch / H Müller / H Michel /  Abstract: Cytochrome c oxidases are among the most important and fundamental enzymes of life. Integrated into membranes they use four electrons from cytochrome c molecules to reduce molecular oxygen (dioxygen) ...Cytochrome c oxidases are among the most important and fundamental enzymes of life. Integrated into membranes they use four electrons from cytochrome c molecules to reduce molecular oxygen (dioxygen) to water. Their catalytic cycle has been considered to start with the oxidized form. Subsequent electron transfers lead to the E-state, the R-state (which binds oxygen), the P-state (with an already split dioxygen bond), the F-state and the O-state again. Here, we determined structures of up to 1.9 Å resolution of these intermediates by single particle cryo-EM. Our results suggest that in the O-state the active site contains a peroxide dianion and in the P-state possibly an intact dioxygen molecule, the F-state may contain a superoxide anion. Thus, the enzyme's catalytic cycle may have to be turned by 180 degrees. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7au6.cif.gz 7au6.cif.gz | 217.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7au6.ent.gz pdb7au6.ent.gz | 166.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7au6.json.gz 7au6.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/au/7au6 https://data.pdbj.org/pub/pdb/validation_reports/au/7au6 ftp://data.pdbj.org/pub/pdb/validation_reports/au/7au6 ftp://data.pdbj.org/pub/pdb/validation_reports/au/7au6 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  11925MC  7ateC  7atnC  7au3C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Cytochrome c oxidase subunit ... , 4 types, 4 molecules ABCD
| #1: Protein | Mass: 62486.645 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Paracoccus denitrificans (bacteria) / References: UniProt: P98002, cytochrome-c oxidase Paracoccus denitrificans (bacteria) / References: UniProt: P98002, cytochrome-c oxidase |
|---|---|
| #2: Protein | Mass: 32563.643 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Paracoccus denitrificans (bacteria) / References: UniProt: P08306, cytochrome-c oxidase Paracoccus denitrificans (bacteria) / References: UniProt: P08306, cytochrome-c oxidase |
| #3: Protein | Mass: 30808.174 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Paracoccus denitrificans (bacteria) / References: UniProt: P06030, cytochrome-c oxidase Paracoccus denitrificans (bacteria) / References: UniProt: P06030, cytochrome-c oxidase |
| #4: Protein/peptide | Mass: 5509.322 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Paracoccus denitrificans (bacteria) / References: UniProt: P77921, cytochrome-c oxidase Paracoccus denitrificans (bacteria) / References: UniProt: P77921, cytochrome-c oxidase |
-Non-polymers , 9 types, 163 molecules 



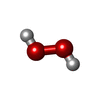












| #5: Chemical | | #6: Chemical | ChemComp-CU / | #7: Chemical | ChemComp-CA / | #8: Chemical | ChemComp-OXY / #9: Chemical | ChemComp-PEO / | #10: Chemical | ChemComp-MN / | #11: Chemical | ChemComp-CUA / | #12: Chemical | #13: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Cytochrome c oxidase with four subunits reconstituted in lipid nanodisc Type: COMPLEX / Entity ID: #1-#4 / Source: NATURAL |
|---|---|
| Molecular weight | Value: 0.127 MDa / Experimental value: NO |
| Source (natural) | Organism:  Paracoccus denitrificans (bacteria) Paracoccus denitrificans (bacteria) |
| Buffer solution | pH: 8 |
| Buffer component | Conc.: 50 mM / Name: Potassium Phosphate / Formula: KPi |
| Specimen | Conc.: 2.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K / Details: 4 seconds before plunging |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Average exposure time: 30 sec. / Electron dose: 30 e/Å2 / Detector mode: COUNTING / Film or detector model: FEI FALCON III (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.18.2_3874: / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 321273 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Space: REAL | ||||||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 3HB3 Accession code: 3HB3 / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller
















 PDBj
PDBj

















