+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6xoh | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of SUMO1-ML00789344 adduct bound to SAE | ||||||
 Components Components |
| ||||||
 Keywords Keywords | LIGASE/LIGASE inhibitor / SAE / SUMO1 / covalent inhibitor / LIGASE / LIGASE-LIGASE inhibitor complex | ||||||
| Function / homology |  Function and homology information Function and homology informationSUMO activating enzyme activity / SUMO activating enzyme complex / negative regulation of potassium ion transmembrane transporter activity / protein localization to nuclear pore / : / SUMOylation of nuclear envelope proteins / ubiquitin activating enzyme activity / SUMO is proteolytically processed / Negative regulation of activity of TFAP2 (AP-2) family transcription factors / SUMO is conjugated to E1 (UBA2:SAE1) ...SUMO activating enzyme activity / SUMO activating enzyme complex / negative regulation of potassium ion transmembrane transporter activity / protein localization to nuclear pore / : / SUMOylation of nuclear envelope proteins / ubiquitin activating enzyme activity / SUMO is proteolytically processed / Negative regulation of activity of TFAP2 (AP-2) family transcription factors / SUMO is conjugated to E1 (UBA2:SAE1) / negative regulation of delayed rectifier potassium channel activity / SUMO is transferred from E1 to E2 (UBE2I, UBC9) / negative regulation of DNA binding / negative regulation of action potential / PML body organization / nuclear stress granule / positive regulation of protein sumoylation / small protein activating enzyme binding / SUMO binding / SUMOylation of immune response proteins / SUMOylation of DNA methylation proteins / SUMOylation of SUMOylation proteins / regulation of calcium ion transmembrane transport / ATP-dependent protein binding / XY body / Maturation of nucleoprotein / SUMOylation of RNA binding proteins / Transferases; Acyltransferases; Aminoacyltransferases / regulation of cardiac muscle cell contraction / Postmitotic nuclear pore complex (NPC) reformation / : / Maturation of nucleoprotein / negative regulation of protein import into nucleus / ubiquitin-like protein conjugating enzyme binding / SUMOylation of ubiquitinylation proteins / ubiquitin-specific protease binding / transcription factor binding / cellular response to cadmium ion / SUMOylation of transcription factors / ubiquitin-like protein ligase binding / roof of mouth development / SUMOylation of DNA replication proteins / protein sumoylation / potassium channel regulator activity / Regulation of IFNG signaling / postsynaptic cytosol / nuclear pore / : / transporter activator activity / SUMOylation of DNA damage response and repair proteins / presynaptic cytosol / Transcriptional and post-translational regulation of MITF-M expression and activity / SUMOylation of transcription cofactors / SUMOylation of chromatin organization proteins / SUMOylation of intracellular receptors / enzyme activator activity / positive regulation of protein-containing complex assembly / PKR-mediated signaling / PML body / regulation of protein stability / protein tag activity / Formation of Incision Complex in GG-NER / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / regulation of protein localization / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / cellular response to heat / transferase activity / nuclear membrane / protein stabilization / nuclear speck / nuclear body / protein heterodimerization activity / DNA repair / negative regulation of DNA-templated transcription / ubiquitin protein ligase binding / nucleolus / glutamatergic synapse / enzyme binding / magnesium ion binding / RNA binding / nucleoplasm / ATP binding / nucleus / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.226 Å MOLECULAR REPLACEMENT / Resolution: 2.226 Å | ||||||
 Authors Authors | Sintchak, M. / Lane, W. / Bump, N. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2021 Journal: J.Med.Chem. / Year: 2021Title: Discovery of TAK-981, a First-in-Class Inhibitor of SUMO-Activating Enzyme for the Treatment of Cancer. Authors: Langston, S.P. / Grossman, S. / England, D. / Afroze, R. / Bence, N. / Bowman, D. / Bump, N. / Chau, R. / Chuang, B.C. / Claiborne, C. / Cohen, L. / Connolly, K. / Duffey, M. / Durvasula, N. ...Authors: Langston, S.P. / Grossman, S. / England, D. / Afroze, R. / Bence, N. / Bowman, D. / Bump, N. / Chau, R. / Chuang, B.C. / Claiborne, C. / Cohen, L. / Connolly, K. / Duffey, M. / Durvasula, N. / Freeze, S. / Gallery, M. / Galvin, K. / Gaulin, J. / Gershman, R. / Greenspan, P. / Grieves, J. / Guo, J. / Gulavita, N. / Hailu, S. / He, X. / Hoar, K. / Hu, Y. / Hu, Z. / Ito, M. / Kim, M.S. / Lane, S.W. / Lok, D. / Lublinsky, A. / Mallender, W. / McIntyre, C. / Minissale, J. / Mizutani, H. / Mizutani, M. / Molchinova, N. / Ono, K. / Patil, A. / Qian, M. / Riceberg, J. / Shindi, V. / Sintchak, M.D. / Song, K. / Soucy, T. / Wang, Y. / Xu, H. / Yang, X. / Zawadzka, A. / Zhang, J. / Pulukuri, S.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6xoh.cif.gz 6xoh.cif.gz | 194.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6xoh.ent.gz pdb6xoh.ent.gz | 142.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6xoh.json.gz 6xoh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xo/6xoh https://data.pdbj.org/pub/pdb/validation_reports/xo/6xoh ftp://data.pdbj.org/pub/pdb/validation_reports/xo/6xoh ftp://data.pdbj.org/pub/pdb/validation_reports/xo/6xoh | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6xogC  6xoiC  1y8rS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-SUMO-activating enzyme subunit ... , 2 types, 2 molecules AB
| #1: Protein | Mass: 38499.789 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SAE1, AOS1, SUA1, UBLE1A / Production host: Homo sapiens (human) / Gene: SAE1, AOS1, SUA1, UBLE1A / Production host:  |
|---|---|
| #2: Protein | Mass: 71314.508 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: UBA2, SAE2, UBLE1B, HRIHFB2115 / Production host: Homo sapiens (human) / Gene: UBA2, SAE2, UBLE1B, HRIHFB2115 / Production host:  References: UniProt: Q9UBT2, Transferases; Acyltransferases; Aminoacyltransferases |
-Protein , 1 types, 1 molecules C
| #3: Protein | Mass: 11575.005 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SUMO1, SMT3C, SMT3H3, UBL1, OK/SW-cl.43 / Production host: Homo sapiens (human) / Gene: SUMO1, SMT3C, SMT3H3, UBL1, OK/SW-cl.43 / Production host:  |
|---|
-Non-polymers , 4 types, 191 molecules 

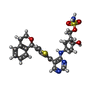




| #4: Chemical | ChemComp-SO4 / |
|---|---|
| #5: Chemical | ChemComp-ZN / |
| #6: Chemical | ChemComp-VB7 / {( |
| #7: Water | ChemComp-HOH / |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.21 Å3/Da / Density % sol: 44.3 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion Details: 50 mM BisTris, pH 6.5, 50 mM Ammonium Sulfate, 30% pentaerythritol ethoxylate (Hampton Index 57) |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  CLSI CLSI  / Beamline: 08ID-1 / Wavelength: 0.9795 Å / Beamline: 08ID-1 / Wavelength: 0.9795 Å |
| Detector | Type: RAYONIX MX-300 / Detector: CCD / Date: Sep 29, 2013 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 2.226→47.004 Å / Num. obs: 50291 / % possible obs: 97.02 % / Redundancy: 3.7 % / Rsym value: 0.064 / Net I/σ(I): 18.48 |
| Reflection shell | Resolution: 2.23→2.31 Å / Mean I/σ(I) obs: 2 / Num. unique obs: 4828 / Rsym value: 0.664 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1Y8R Resolution: 2.226→43.31 Å / Cor.coef. Fo:Fc: 0.96 / Cor.coef. Fo:Fc free: 0.933 / SU B: 6.729 / SU ML: 0.166 / Cross valid method: FREE R-VALUE / ESU R: 0.252 / ESU R Free: 0.209 Details: Hydrogens have been added in their riding positions
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK BULK SOLVENT | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 63.594 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.226→43.31 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller













 PDBj
PDBj




















