[English] 日本語
 Yorodumi
Yorodumi- PDB-6x94: An orthogonal seryl-tRNA synthetase/tRNA pair for noncanonical am... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6x94 | ||||||
|---|---|---|---|---|---|---|---|
| Title | An orthogonal seryl-tRNA synthetase/tRNA pair for noncanonical amino acid mutagenesis in Escherichia coli | ||||||
 Components Components | Serine--tRNA ligase | ||||||
 Keywords Keywords | LIGASE / Serine tRNA-ligase / Protein translation / Serine aminoacylation / Non-hydrolyzable Serine-AMP | ||||||
| Function / homology |  Function and homology information Function and homology informationselenocysteine biosynthetic process / serine-tRNA ligase / serine-tRNA ligase activity / seryl-tRNA aminoacylation / ATP binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  Methanosarcina mazei (archaea) Methanosarcina mazei (archaea) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.45 Å molecular replacement / Resolution: 1.45 Å | ||||||
 Authors Authors | Zambaldo, C. / Koh, M. / Nasertorabi, F. / Han, G.W. / Chatterjee, A. / Stevens, R.C. / Schultz, P.G. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Bioorg.Med.Chem. / Year: 2020 Journal: Bioorg.Med.Chem. / Year: 2020Title: An orthogonal seryl-tRNA synthetase/tRNA pair for noncanonical amino acid mutagenesis in Escherichia coli. Authors: Zambaldo, C. / Koh, M. / Nasertorabi, F. / Han, G.W. / Chatterjee, A. / Stevens, R.C. / Schultz, P.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6x94.cif.gz 6x94.cif.gz | 202.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6x94.ent.gz pdb6x94.ent.gz | 156.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6x94.json.gz 6x94.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6x94_validation.pdf.gz 6x94_validation.pdf.gz | 807.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6x94_full_validation.pdf.gz 6x94_full_validation.pdf.gz | 810.9 KB | Display | |
| Data in XML |  6x94_validation.xml.gz 6x94_validation.xml.gz | 25.6 KB | Display | |
| Data in CIF |  6x94_validation.cif.gz 6x94_validation.cif.gz | 40.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/x9/6x94 https://data.pdbj.org/pub/pdb/validation_reports/x9/6x94 ftp://data.pdbj.org/pub/pdb/validation_reports/x9/6x94 ftp://data.pdbj.org/pub/pdb/validation_reports/x9/6x94 | HTTPS FTP |
-Related structure data
| Related structure data |  2dq3S S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| |||||||||||||||
| Unit cell |
| |||||||||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 48659.363 Da / Num. of mol.: 1 / Mutation: E402V E416V K419P V420E Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Methanosarcina mazei (strain ATCC BAA-159 / DSM 3647 / Goe1 / Go1 / JCM 11833 / OCM 88) (archaea) Methanosarcina mazei (strain ATCC BAA-159 / DSM 3647 / Goe1 / Go1 / JCM 11833 / OCM 88) (archaea)Strain: ATCC BAA-159 / DSM 3647 / Goe1 / Go1 / JCM 11833 / OCM 88 Gene: serS, MM_0865 / Plasmid: pET22b Details (production host): T7 RNA polimerase (IPTG indicuble expression system), Ampicillin resistant Production host:  |
|---|
-Non-polymers , 7 types, 695 molecules 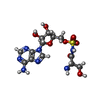











| #2: Chemical | ChemComp-SSA / | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
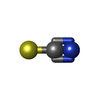
| #3: Chemical | | #4: Chemical | ChemComp-DMS / | #5: Chemical | ChemComp-SCN / #6: Chemical | ChemComp-K / | #7: Chemical | ChemComp-CL / | #8: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.71 Å3/Da / Density % sol: 54.6 % / Description: Large, rectangular 20 x 30 x (50-300) um |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, hanging drop / pH: 7 / Details: 18% PEG3350, 200 mM thiocyanate / PH range: 6.5 - 7.5 |
-Data collection
| Diffraction | Mean temperature: 100 K / Ambient temp details: Cryo / Serial crystal experiment: N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL12-2 / Wavelength: 0.97946 Å / Beamline: BL12-2 / Wavelength: 0.97946 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Mar 6, 2018 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: double crystal Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.97946 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.45→79.707 Å / Num. obs: 86540 / % possible obs: 99.9 % / Redundancy: 9.9 % / CC1/2: 1 / Rmerge(I) obs: 0.06 / Rpim(I) all: 0.02 / Rrim(I) all: 0.063 / Rsym value: 0.06 / Net I/av σ(I): 8.8 / Net I/σ(I): 18.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 2DQ3 Resolution: 1.45→29.22 Å / Cor.coef. Fo:Fc: 0.98 / Cor.coef. Fo:Fc free: 0.969 / SU B: 2.051 / SU ML: 0.04 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.054 / ESU R Free: 0.056 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : WITH TLS ADDED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 98.21 Å2 / Biso mean: 22.611 Å2 / Biso min: 12.56 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.45→29.22 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.45→1.488 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj







