+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6v3c | ||||||
|---|---|---|---|---|---|---|---|
| Title | K2P2.1(TREK-1)I110D:Ru360 bound channel structure | ||||||
 Components Components | Potassium channel subfamily K member 2 | ||||||
 Keywords Keywords | MEMBRANE PROTEIN / K channel / TREK-1 / Mus musculus | ||||||
| Function / homology |  Function and homology information Function and homology informationTWIK related potassium channel (TREK) / Phase 4 - resting membrane potential / cellular response to arachidonate / mechanosensitive potassium channel activity / negative regulation of DNA biosynthetic process / detection of mechanical stimulus involved in sensory perception of touch / chemical synaptic transmission, postsynaptic / stabilization of membrane potential / cardiac ventricle development / ligand-gated channel activity ...TWIK related potassium channel (TREK) / Phase 4 - resting membrane potential / cellular response to arachidonate / mechanosensitive potassium channel activity / negative regulation of DNA biosynthetic process / detection of mechanical stimulus involved in sensory perception of touch / chemical synaptic transmission, postsynaptic / stabilization of membrane potential / cardiac ventricle development / ligand-gated channel activity / negative regulation of cardiac muscle cell proliferation / positive regulation of cellular response to hypoxia / potassium ion leak channel activity / node of Ranvier / outward rectifier potassium channel activity / glutamate secretion / astrocyte projection / regulation of synaptic transmission, GABAergic / cochlea development / voltage-gated potassium channel activity / response to axon injury / neuronal action potential / voltage-gated potassium channel complex / chloride transmembrane transport / calyx of Held / regulation of membrane potential / potassium ion transport / sarcolemma / postsynaptic density membrane / Schaffer collateral - CA1 synapse / memory / cellular response to hypoxia / apical plasma membrane / G protein-coupled receptor signaling pathway / protein heterodimerization activity / neuronal cell body / dendrite / cell surface / metal ion binding / identical protein binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.51 Å MOLECULAR REPLACEMENT / Resolution: 3.51 Å | ||||||
 Authors Authors | Pope, L. / Lolicato, M. / Minor, D.L. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Cell Chem Biol / Year: 2020 Journal: Cell Chem Biol / Year: 2020Title: Polynuclear Ruthenium Amines Inhibit K2PChannels via a "Finger in the Dam" Mechanism. Authors: Pope, L. / Lolicato, M. / Minor Jr., D.L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6v3c.cif.gz 6v3c.cif.gz | 117.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6v3c.ent.gz pdb6v3c.ent.gz | 88.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6v3c.json.gz 6v3c.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/v3/6v3c https://data.pdbj.org/pub/pdb/validation_reports/v3/6v3c ftp://data.pdbj.org/pub/pdb/validation_reports/v3/6v3c ftp://data.pdbj.org/pub/pdb/validation_reports/v3/6v3c | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6v36C  6v37C  6v3iC  4rueS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 34305.867 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Komagataella pastoris (fungus) / References: UniProt: P97438*PLUS Komagataella pastoris (fungus) / References: UniProt: P97438*PLUS |
|---|
-Non-polymers , 7 types, 17 molecules 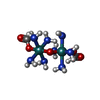



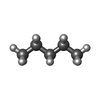





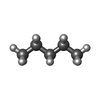


| #2: Chemical | ChemComp-RU3 / | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #3: Chemical | | #4: Chemical | ChemComp-K / #5: Chemical | ChemComp-OCT / #6: Chemical | ChemComp-LNK / | #7: Chemical | ChemComp-HEX / | #8: Water | ChemComp-HOH / | |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.79 Å3/Da / Density % sol: 67.56 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop Details: 0.1 M KCl, 1-3 mM CdCl2, 0.1 M HEPES 7-8, 20-25% PEG400 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 23-ID-B / Wavelength: 1.0332 Å / Beamline: 23-ID-B / Wavelength: 1.0332 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Aug 18, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.0332 Å / Relative weight: 1 |
| Reflection | Resolution: 3.51→46.4 Å / Num. obs: 12595 / % possible obs: 100 % / Redundancy: 13.3 % / CC1/2: 1 / Net I/σ(I): 10.8 |
| Reflection shell | Resolution: 3.51→3.85 Å / Num. unique obs: 530 / CC1/2: 0.149 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4RUE Resolution: 3.51→14.98 Å / SU ML: 0.6 / Cross valid method: THROUGHOUT / σ(F): 1.33 / Phase error: 44.65 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 327.88 Å2 / Biso mean: 203.5724 Å2 / Biso min: 119.59 Å2 | |||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 3.51→14.98 Å
| |||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 4
|
 Movie
Movie Controller
Controller











 PDBj
PDBj









