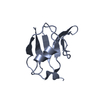
Entry Database : PDB / ID : 6ts3Title EF-hands 3 and 4 of alpha-actinin in complex with CaMKII regulatory segment ACE-ASN-ALA-ARG-ARG-LYS-LEU-LYS-GLY-ALA-ILE-LEU-THR-THR-MET-LEU-ALA-THR-ARG-ASN-PHE Alpha-actinin-2 Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Mus musculus (house mouse)Method / / / Resolution : 1.28 Å Authors Zhu, J. / Gold, M. Funding support Organization Grant number Country Biotechnology and Biological Sciences Research Council (BBSRC) 533303
Journal : To Be Published Title : EF-hands 3 and 4 of alpha-actinin in complex with CaMKII regulatory segmentAuthors : Zhu, J. / Gold, M. History Deposition Dec 19, 2019 Deposition site / Processing site Revision 1.0 Jan 13, 2021 Provider / Type Revision 1.1 Apr 9, 2025 Group / Database references / Structure summaryCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details Item / _database_2.pdbx_database_accession / _pdbx_entry_details.has_protein_modification
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human)
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  SAD / Resolution: 1.28 Å
SAD / Resolution: 1.28 Å  Authors
Authors United Kingdom, 1items
United Kingdom, 1items  Citation
Citation Journal: To Be Published
Journal: To Be Published Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6ts3.cif.gz
6ts3.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6ts3.ent.gz
pdb6ts3.ent.gz PDB format
PDB format 6ts3.json.gz
6ts3.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ts/6ts3
https://data.pdbj.org/pub/pdb/validation_reports/ts/6ts3 ftp://data.pdbj.org/pub/pdb/validation_reports/ts/6ts3
ftp://data.pdbj.org/pub/pdb/validation_reports/ts/6ts3 Links
Links Assembly
Assembly


 Components
Components Homo sapiens (human) / Gene: ACTN2 / Production host:
Homo sapiens (human) / Gene: ACTN2 / Production host: 

 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  Diamond
Diamond  / Beamline: I04 / Wavelength: 0.9795 Å
/ Beamline: I04 / Wavelength: 0.9795 Å Processing
Processing SAD / Resolution: 1.28→44.46 Å / SU ML: 0.1249 / Cross valid method: FREE R-VALUE / σ(F): 1.37 / Phase error: 22.6175
SAD / Resolution: 1.28→44.46 Å / SU ML: 0.1249 / Cross valid method: FREE R-VALUE / σ(F): 1.37 / Phase error: 22.6175  Movie
Movie Controller
Controller









 PDBj
PDBj

















