[English] 日本語
 Yorodumi
Yorodumi- PDB-6tqr: The crystal structure of the MSP domain of human VAP-A in complex... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6tqr | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | The crystal structure of the MSP domain of human VAP-A in complex with the Phospho-FFAT motif of STARD3. | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords | PROTEIN BINDING / Membrane contact sites / FFAT motif / MSP domain / Endoplasmic reticulum | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationvesicle tethering to endoplasmic reticulum / progesterone biosynthetic process / endoplasmic reticulum-endosome membrane contact site / FFAT motif binding / endoplasmic reticulum-plasma membrane tethering / sphingomyelin biosynthetic process / organelle membrane contact site / ceramide transport / COPII-coated vesicle budding / host-mediated suppression of viral genome replication ...vesicle tethering to endoplasmic reticulum / progesterone biosynthetic process / endoplasmic reticulum-endosome membrane contact site / FFAT motif binding / endoplasmic reticulum-plasma membrane tethering / sphingomyelin biosynthetic process / organelle membrane contact site / ceramide transport / COPII-coated vesicle budding / host-mediated suppression of viral genome replication / sterol transport / host-mediated activation of viral genome replication / protein localization to endoplasmic reticulum / Sphingolipid de novo biosynthesis / Pregnenolone biosynthesis / phospholipid transport / cholesterol transfer activity / cholesterol transport / azurophil granule membrane / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / cholesterol binding / mitochondrial transport / steroid metabolic process / viral release from host cell / bicellular tight junction / endoplasmic reticulum to Golgi vesicle-mediated transport / cholesterol metabolic process / protein-membrane adaptor activity / lipid metabolic process / neuron projection development / late endosome membrane / microtubule cytoskeleton / nuclear membrane / microtubule binding / vesicle / membrane fusion / positive regulation of canonical NF-kappaB signal transduction / endosome / cadherin binding / protein heterodimerization activity / Golgi membrane / protein domain specific binding / lysosomal membrane / Neutrophil degranulation / endoplasmic reticulum membrane / endoplasmic reticulum / protein homodimerization activity / mitochondrion / nucleoplasm / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.85 Å molecular replacement / Resolution: 1.85 Å | |||||||||||||||
 Authors Authors | McEwen, A.G. / Poussin-Courmontagne, P. / Di Mattia, T. / Wendling, C. / Cavarelli, J. / Tomasetto, C. / Alpy, F. | |||||||||||||||
| Funding support |  France, 4items France, 4items
| |||||||||||||||
 Citation Citation |  Journal: Embo J. / Year: 2020 Journal: Embo J. / Year: 2020Title: FFAT motif phosphorylation controls formation and lipid transfer function of inter-organelle contacts. Authors: Di Mattia, T. / Martinet, A. / Ikhlef, S. / McEwen, A.G. / Nomine, Y. / Wendling, C. / Poussin-Courmontagne, P. / Voilquin, L. / Eberling, P. / Ruffenach, F. / Cavarelli, J. / Slee, J. / ...Authors: Di Mattia, T. / Martinet, A. / Ikhlef, S. / McEwen, A.G. / Nomine, Y. / Wendling, C. / Poussin-Courmontagne, P. / Voilquin, L. / Eberling, P. / Ruffenach, F. / Cavarelli, J. / Slee, J. / Levine, T.P. / Drin, G. / Tomasetto, C. / Alpy, F. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6tqr.cif.gz 6tqr.cif.gz | 237.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6tqr.ent.gz pdb6tqr.ent.gz | 187.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6tqr.json.gz 6tqr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tq/6tqr https://data.pdbj.org/pub/pdb/validation_reports/tq/6tqr ftp://data.pdbj.org/pub/pdb/validation_reports/tq/6tqr ftp://data.pdbj.org/pub/pdb/validation_reports/tq/6tqr | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6tqsC  6tqtC  6tquC  1z9oS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 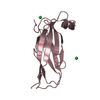
| ||||||||
| 4 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 24478.170 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Details: MSP domain / Source: (gene. exp.)  Homo sapiens (human) / Gene: VAPA, VAP33 / Production host: Homo sapiens (human) / Gene: VAPA, VAP33 / Production host:  #2: Protein/peptide | Mass: 1983.762 Da / Num. of mol.: 2 / Source method: obtained synthetically / Details: phospho-FFAT motif / Source: (synth.)  Homo sapiens (human) / References: UniProt: Q14849 Homo sapiens (human) / References: UniProt: Q14849#3: Chemical | ChemComp-CL / #4: Water | ChemComp-HOH / | Has ligand of interest | N | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.21 Å3/Da / Density % sol: 44.4 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 7.5 / Details: 25% PEG 2000 MME, 0.1 M HEPES pH 7.5 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-1 / Wavelength: 0.98 Å / Beamline: ID23-1 / Wavelength: 0.98 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Apr 25, 2015 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.98 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.85→42.26 Å / Num. obs: 42485 / % possible obs: 92.8 % / Redundancy: 3.338 % / Biso Wilson estimate: 38.514 Å2 / CC1/2: 0.99 / Rmerge(I) obs: 0.208 / Rpim(I) all: 0.133 / Rrim(I) all: 0.248 / Χ2: 1.116 / Net I/σ(I): 4.57 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1Z9O Resolution: 1.85→42.26 Å / SU ML: 0.27 / Cross valid method: THROUGHOUT / σ(F): 1.92 / Phase error: 34.68
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 149.49 Å2 / Biso mean: 36.8225 Å2 / Biso min: 8.91 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.85→42.26 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 9
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj





