[English] 日本語
 Yorodumi
Yorodumi- PDB-6swc: IC2B model of cryo-EM structure of a full archaeal ribosomal tran... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6swc | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | IC2B model of cryo-EM structure of a full archaeal ribosomal translation initiation complex devoid of aIF1 in P. abyssi | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | RIBOSOME / Translation initiation / cryo-EM / tRNA / evolution / archaea / rRNA modifications | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationformation of translation preinitiation complex / ribonuclease P activity / tRNA 5'-leader removal / protein-synthesizing GTPase / translation elongation factor activity / translation initiation factor activity / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / maturation of SSU-rRNA / translational initiation / ribosome biogenesis ...formation of translation preinitiation complex / ribonuclease P activity / tRNA 5'-leader removal / protein-synthesizing GTPase / translation elongation factor activity / translation initiation factor activity / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / maturation of SSU-rRNA / translational initiation / ribosome biogenesis / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / cytoplasmic translation / tRNA binding / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / GTPase activity / GTP binding / RNA binding / zinc ion binding / metal ion binding / cytoplasm / cytosol Similarity search - Function | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |    Pyrococcus abyssi (archaea) Pyrococcus abyssi (archaea)  Pyrococcus abyssi GE5 (archaea) Pyrococcus abyssi GE5 (archaea)  Saccharolobus solfataricus (archaea) Saccharolobus solfataricus (archaea) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.3 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Coureux, P.-D. / Mechulam, Y. / Schmitt, E. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  France, 1items France, 1items
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2020 Journal: Commun Biol / Year: 2020Title: Cryo-EM study of an archaeal 30S initiation complex gives insights into evolution of translation initiation. Authors: Pierre-Damien Coureux / Christine Lazennec-Schurdevin / Sophie Bourcier / Yves Mechulam / Emmanuelle Schmitt /  Abstract: Archaeal translation initiation occurs within a macromolecular complex containing the small ribosomal subunit (30S) bound to mRNA, initiation factors aIF1, aIF1A and the ternary complex aIF2:GDPNP: ...Archaeal translation initiation occurs within a macromolecular complex containing the small ribosomal subunit (30S) bound to mRNA, initiation factors aIF1, aIF1A and the ternary complex aIF2:GDPNP:Met-tRNA. Here, we determine the cryo-EM structure of a 30S:mRNA:aIF1A:aIF2:GTP:Met-tRNA complex from Pyrococcus abyssi at 3.2 Å resolution. It highlights archaeal features in ribosomal proteins and rRNA modifications. We find an aS21 protein, at the location of eS21 in eukaryotic ribosomes. Moreover, we identify an N-terminal extension of archaeal eL41 contacting the P site. We characterize 34 N-acetylcytidines distributed throughout 16S rRNA, likely contributing to hyperthermostability. Without aIF1, the 30S head is stabilized and initiator tRNA is tightly bound to the P site. A network of interactions involving tRNA, mRNA, rRNA modified nucleotides and C-terminal tails of uS9, uS13 and uS19 is observed. Universal features and domain-specific idiosyncrasies of translation initiation are discussed in light of ribosomal structures from representatives of each domain of life. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6swc.cif.gz 6swc.cif.gz | 1.6 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6swc.ent.gz pdb6swc.ent.gz | 1.2 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6swc.json.gz 6swc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sw/6swc https://data.pdbj.org/pub/pdb/validation_reports/sw/6swc ftp://data.pdbj.org/pub/pdb/validation_reports/sw/6swc ftp://data.pdbj.org/pub/pdb/validation_reports/sw/6swc | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  10322MC  6sw9C  6swdC  6sweC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-RNA chain , 3 types, 3 molecules 254
| #1: RNA chain | Mass: 487700.312 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)   Pyrococcus abyssi GE5 (archaea) Pyrococcus abyssi GE5 (archaea) |
|---|---|
| #30: RNA chain | Mass: 6448.871 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)   Pyrococcus abyssi GE5 (archaea) Pyrococcus abyssi GE5 (archaea) |
| #31: RNA chain | Mass: 24504.695 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
+30S ribosomal protein ... , 26 types, 26 molecules ABDEFGHIJKLMNOPQRSTUVWXYZ0
-Protein , 3 types, 3 molecules C36
| #4: Protein | Mass: 7163.395 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)   Pyrococcus abyssi (strain GE5 / Orsay) (archaea) Pyrococcus abyssi (strain GE5 / Orsay) (archaea)Strain: GE5 / Orsay / References: UniProt: G8ZFK7 |
|---|---|
| #29: Protein | Mass: 13442.678 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)   Pyrococcus abyssi (strain GE5 / Orsay) (archaea) Pyrococcus abyssi (strain GE5 / Orsay) (archaea)Strain: GE5 / Orsay / References: UniProt: P62008 |
| #32: Protein | Mass: 13078.265 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Pyrococcus abyssi (strain GE5 / Orsay) (archaea) Pyrococcus abyssi (strain GE5 / Orsay) (archaea)Strain: GE5 / Orsay / Gene: eIF1A, aif1A, PYRAB05910, PAB2441 / Production host:  |
-Translation initiation factor 2 subunit ... , 3 types, 3 molecules 789
| #33: Protein | Mass: 45849.230 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: Model from high resolution structures of aIF2 gamma of Saccharolobus solfataricus Source: (gene. exp.)   Pyrococcus abyssi GE5 (archaea) / Production host: Pyrococcus abyssi GE5 (archaea) / Production host:  |
|---|---|
| #34: Protein | Mass: 15942.740 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: Model from high resolution structures of aIF2 beta of Saccharolobus solfataricus Source: (gene. exp.)   Pyrococcus abyssi GE5 (archaea) / Production host: Pyrococcus abyssi GE5 (archaea) / Production host:  |
| #35: Protein | Mass: 30432.355 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: Model from high resolution structures of aIF2 alpha of Saccharolobus solfataricus Source: (gene. exp.)   Saccharolobus solfataricus (strain ATCC 35092 / DSM 1617 / JCM 11322 / P2) (archaea) Saccharolobus solfataricus (strain ATCC 35092 / DSM 1617 / JCM 11322 / P2) (archaea)Strain: ATCC 35092 / DSM 1617 / JCM 11322 / P2 / Gene: eif2a, aif2a, SSO1050 / Production host:  |
-Non-polymers , 5 types, 84 molecules 

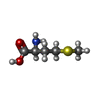






| #36: Chemical | ChemComp-MG / #37: Chemical | ChemComp-ZN / #38: Chemical | ChemComp-MET / | #39: Chemical | ChemComp-GNP / | #40: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 1.05 MDa / Experimental value: NO | ||||||||||||||||||||||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 6.7 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 2 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.15.2_3472: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 142000 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj




































