+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 6rzu | ||||||
|---|---|---|---|---|---|---|---|
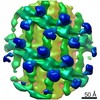
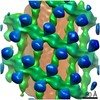
| タイトル | Structure of s-Mgm1 decorating the outer surface of tubulated lipid membranes in the GTPgammaS bound state | ||||||
 要素 要素 | Putative mitochondrial dynamin protein | ||||||
 キーワード キーワード | CONTRACTILE PROTEIN / mitochondrial protein / membrane remodelling / GTPase / mitochondrial dynamics / membrane tubulation | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報membrane bending activity / GTPase-dependent fusogenic activity / dynamin GTPase / receptor internalization / mitochondrial intermembrane space / microtubule binding / microtubule / mitochondrial inner membrane / GTPase activity / lipid binding ...membrane bending activity / GTPase-dependent fusogenic activity / dynamin GTPase / receptor internalization / mitochondrial intermembrane space / microtubule binding / microtubule / mitochondrial inner membrane / GTPase activity / lipid binding / GTP binding / metal ion binding / plasma membrane 類似検索 - 分子機能 | ||||||
| 生物種 |  Chaetomium thermophilum var. thermophilum DSM 1495 (菌類) Chaetomium thermophilum var. thermophilum DSM 1495 (菌類) | ||||||
| 手法 | 電子顕微鏡法 / サブトモグラム平均法 / クライオ電子顕微鏡法 / 解像度: 14.7 Å | ||||||
 データ登録者 データ登録者 | Faelber, K. / Dietrich, L. / Noel, J.K. / Sanchez, R. / Kudryashev, M. / Kuelbrandt, W. / Daumke, O. | ||||||
| 資金援助 |  ドイツ, 1件 ドイツ, 1件
| ||||||
 引用 引用 |  ジャーナル: Nature / 年: 2019 ジャーナル: Nature / 年: 2019タイトル: Structure and assembly of the mitochondrial membrane remodelling GTPase Mgm1. 著者: Katja Faelber / Lea Dietrich / Jeffrey K Noel / Florian Wollweber / Anna-Katharina Pfitzner / Alexander Mühleip / Ricardo Sánchez / Misha Kudryashev / Nicolas Chiaruttini / Hauke Lilie / ...著者: Katja Faelber / Lea Dietrich / Jeffrey K Noel / Florian Wollweber / Anna-Katharina Pfitzner / Alexander Mühleip / Ricardo Sánchez / Misha Kudryashev / Nicolas Chiaruttini / Hauke Lilie / Jeanette Schlegel / Eva Rosenbaum / Manuel Hessenberger / Claudia Matthaeus / Séverine Kunz / Alexander von der Malsburg / Frank Noé / Aurélien Roux / Martin van der Laan / Werner Kühlbrandt / Oliver Daumke /   要旨: Balanced fusion and fission are key for the proper function and physiology of mitochondria. Remodelling of the mitochondrial inner membrane is mediated by the dynamin-like protein mitochondrial ...Balanced fusion and fission are key for the proper function and physiology of mitochondria. Remodelling of the mitochondrial inner membrane is mediated by the dynamin-like protein mitochondrial genome maintenance 1 (Mgm1) in fungi or the related protein optic atrophy 1 (OPA1) in animals. Mgm1 is required for the preservation of mitochondrial DNA in yeast, whereas mutations in the OPA1 gene in humans are a common cause of autosomal dominant optic atrophy-a genetic disorder that affects the optic nerve. Mgm1 and OPA1 are present in mitochondria as a membrane-integral long form and a short form that is soluble in the intermembrane space. Yeast strains that express temperature-sensitive mutants of Mgm1 or mammalian cells that lack OPA1 display fragmented mitochondria, which suggests that Mgm1 and OPA1 have an important role in inner-membrane fusion. Consistently, only the mitochondrial outer membrane-not the inner membrane-fuses in the absence of functional Mgm1. Mgm1 and OPA1 have also been shown to maintain proper cristae architecture; for example, OPA1 prevents the release of pro-apoptotic factors by tightening crista junctions. Finally, the short form of OPA1 localizes to mitochondrial constriction sites, where it presumably promotes mitochondrial fission. How Mgm1 and OPA1 perform their diverse functions in membrane fusion, scission and cristae organization is at present unknown. Here we present crystal and electron cryo-tomography structures of Mgm1 from Chaetomium thermophilum. Mgm1 consists of a GTPase (G) domain, a bundle signalling element domain, a stalk, and a paddle domain that contains a membrane-binding site. Biochemical and cell-based experiments demonstrate that the Mgm1 stalk mediates the assembly of bent tetramers into helical filaments. Electron cryo-tomography studies of Mgm1-decorated lipid tubes and fluorescence microscopy experiments on reconstituted membrane tubes indicate how the tetramers assemble on positively or negatively curved membranes. Our findings convey how Mgm1 and OPA1 filaments dynamically remodel the mitochondrial inner membrane. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  6rzu.cif.gz 6rzu.cif.gz | 1.1 MB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb6rzu.ent.gz pdb6rzu.ent.gz | 912.4 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  6rzu.json.gz 6rzu.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  6rzu_validation.pdf.gz 6rzu_validation.pdf.gz | 1.4 MB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  6rzu_full_validation.pdf.gz 6rzu_full_validation.pdf.gz | 1.8 MB | 表示 | |
| XML形式データ |  6rzu_validation.xml.gz 6rzu_validation.xml.gz | 242.1 KB | 表示 | |
| CIF形式データ |  6rzu_validation.cif.gz 6rzu_validation.cif.gz | 364.1 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/rz/6rzu https://data.pdbj.org/pub/pdb/validation_reports/rz/6rzu ftp://data.pdbj.org/pub/pdb/validation_reports/rz/6rzu ftp://data.pdbj.org/pub/pdb/validation_reports/rz/6rzu | HTTPS FTP |
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
|
|---|---|
| 1 |
|
- 要素
要素
| #1: タンパク質 | 分子量: 77360.172 Da / 分子数: 12 / 由来タイプ: 組換発現 由来: (組換発現)  Chaetomium thermophilum var. thermophilum DSM 1495 (菌類) Chaetomium thermophilum var. thermophilum DSM 1495 (菌類)遺伝子: CTHT_0065850 / 発現宿主:  Has protein modification | Y | |
|---|
-実験情報
-実験
| 実験 | 手法: 電子顕微鏡法 |
|---|---|
| EM実験 | 試料の集合状態: PARTICLE / 3次元再構成法: サブトモグラム平均法 |
- 試料調製
試料調製
| 構成要素 | 名称: Mgm1 / タイプ: COMPLEX / 詳細: short isoform with C- and N-terminal truncations / Entity ID: all / 由来: RECOMBINANT |
|---|---|
| 分子量 | 実験値: NO |
| 由来(天然) | 生物種:  Chaetomium thermophilum var. thermophilum DSM 1495 (菌類) Chaetomium thermophilum var. thermophilum DSM 1495 (菌類) |
| 由来(組換発現) | 生物種:  |
| 緩衝液 | pH: 7.4 / 詳細: 20 mM HEPES, 200 mM NaCl, residual MgCl2, 9mM KCl. |
| 試料 | 濃度: 5 mg/ml / 包埋: NO / シャドウイング: NO / 染色: NO / 凍結: YES 詳細: Sample was prepared in the described buffer. Just before freezing 6 nm colloidal gold fiducial marker were added to the sample in a 1:1 ratio. |
| 急速凍結 | 装置: FEI VITROBOT MARK IV / 凍結剤: ETHANE / 湿度: 70 % / 凍結前の試料温度: 277.15 K |
- 電子顕微鏡撮影
電子顕微鏡撮影
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
|---|---|
| 顕微鏡 | モデル: FEI TITAN KRIOS |
| 電子銃 | 電子線源:  FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM |
| 電子レンズ | モード: BRIGHT FIELD / 倍率(公称値): 53000 X / 最大 デフォーカス(公称値): 4000 nm / 最小 デフォーカス(公称値): 2000 nm / Cs: 2.7 mm / C2レンズ絞り径: 70 µm / アライメント法: BASIC |
| 試料ホルダ | 凍結剤: NITROGEN 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER |
| 撮影 | 電子線照射量: 2 e/Å2 / 検出モード: COUNTING フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) |
| 電子光学装置 | エネルギーフィルタースリット幅: 20 eV |
- 解析
解析
| EMソフトウェア |
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF補正 | 詳細: CTF determination was done by Gctf and correction was performed by ctfphaseflip from Imod タイプ: PHASE FLIPPING ONLY | ||||||||||||||||||||||||||||||||||||||||
| 対称性 | 点対称性: C1 (非対称) | ||||||||||||||||||||||||||||||||||||||||
| 3次元再構成 | 解像度: 14.7 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 粒子像の数: 9471 / アルゴリズム: BACK PROJECTION 詳細: Half sets were generated not even-odd but as upper and lower-halves of particles in given tomogram クラス平均像の数: 1 / 対称性のタイプ: POINT | ||||||||||||||||||||||||||||||||||||||||
| EM volume selection | 手法: Geometry-assisted particle picking form tube surfaces 詳細: with the use of Dynamo Catalogue system / Num. of tomograms: 12 / Num. of volumes extracted: 71884 Reference model: global average of the particles rotated to the known initial orientations |
 ムービー
ムービー コントローラー
コントローラー













 PDBj
PDBj