[English] 日本語
 Yorodumi
Yorodumi- PDB-6rv4: Crystal structure of the human two pore domain potassium ion chan... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6rv4 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
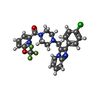
| Title | Crystal structure of the human two pore domain potassium ion channel TASK-1 (K2P3.1) in a closed conformation with a bound inhibitor BAY 2341237 | ||||||||||||
 Components Components | Potassium channel subfamily K member 3 | ||||||||||||
 Keywords Keywords | MEMBRANE PROTEIN / potassium channel / Structural Genomics / Structural Genomics Consortium / SGC | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationopen rectifier potassium channel activity / detection of hypoxic conditions in blood by carotid body chemoreceptor signaling / regulation of action potential firing rate / TWIK-releated acid-sensitive K+ channel (TASK) / Phase 4 - resting membrane potential / potassium ion leak channel activity / regulation of resting membrane potential / cellular response to acidic pH / S100 protein binding / outward rectifier potassium channel activity ...open rectifier potassium channel activity / detection of hypoxic conditions in blood by carotid body chemoreceptor signaling / regulation of action potential firing rate / TWIK-releated acid-sensitive K+ channel (TASK) / Phase 4 - resting membrane potential / potassium ion leak channel activity / regulation of resting membrane potential / cellular response to acidic pH / S100 protein binding / outward rectifier potassium channel activity / negative regulation of cytosolic calcium ion concentration / cellular response to zinc ion / sodium channel activity / monoatomic ion channel activity / cochlea development / potassium channel activity / potassium ion transmembrane transport / potassium ion transport / monoatomic ion transmembrane transport / cellular response to hypoxia / chemical synaptic transmission / response to xenobiotic stimulus / protein heterodimerization activity / synapse / metal ion binding / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.1 Å MOLECULAR REPLACEMENT / Resolution: 3.1 Å | ||||||||||||
 Authors Authors | Rodstrom, K.E.J. / Pike, A.C.W. / Zhang, W. / Quigley, A. / Speedman, D. / Mukhopadhyay, S.M.M. / Shrestha, L. / Chalk, R. / Venkaya, S. / Bushell, S.R. ...Rodstrom, K.E.J. / Pike, A.C.W. / Zhang, W. / Quigley, A. / Speedman, D. / Mukhopadhyay, S.M.M. / Shrestha, L. / Chalk, R. / Venkaya, S. / Bushell, S.R. / Tessitore, A. / Burgess-Brown, N. / Arrowsmith, C.H. / Edwards, A.M. / Bountra, C. / Carpenter, E.P. / Structural Genomics Consortium (SGC) | ||||||||||||
| Funding support |  United Kingdom, 3items United Kingdom, 3items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: A lower X-gate in TASK channels traps inhibitors within the vestibule. Authors: Rodstrom, K.E.J. / Kiper, A.K. / Zhang, W. / Rinne, S. / Pike, A.C.W. / Goldstein, M. / Conrad, L.J. / Delbeck, M. / Hahn, M.G. / Meier, H. / Platzk, M. / Quigley, A. / Speedman, D. / ...Authors: Rodstrom, K.E.J. / Kiper, A.K. / Zhang, W. / Rinne, S. / Pike, A.C.W. / Goldstein, M. / Conrad, L.J. / Delbeck, M. / Hahn, M.G. / Meier, H. / Platzk, M. / Quigley, A. / Speedman, D. / Shrestha, L. / Mukhopadhyay, S.M.M. / Burgess-Brown, N.A. / Tucker, S.J. / Muller, T. / Decher, N. / Carpenter, E.P. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6rv4.cif.gz 6rv4.cif.gz | 424.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6rv4.ent.gz pdb6rv4.ent.gz | 348.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6rv4.json.gz 6rv4.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/rv/6rv4 https://data.pdbj.org/pub/pdb/validation_reports/rv/6rv4 ftp://data.pdbj.org/pub/pdb/validation_reports/rv/6rv4 ftp://data.pdbj.org/pub/pdb/validation_reports/rv/6rv4 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6rv2C  6rv3C  4bw5S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 30117.014 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Details: Two-pore domain potassium channel K2P3.1 (TASK-1) / Source: (gene. exp.)  Homo sapiens (human) / Gene: KCNK3, TASK, TASK1 / Production host: Homo sapiens (human) / Gene: KCNK3, TASK, TASK1 / Production host:  #2: Chemical | ChemComp-K / #3: Chemical | ChemComp-Y01 / #4: Chemical | #5: Chemical | ChemComp-PC1 / |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 4.64 Å3/Da / Density % sol: 73.49 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 8.5 Details: 0.1 M TRIS pH 8.5, 0.05 M KCl, 31% v/v PEG400, 3% w/v sucrose Temp details: Ambient |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I24 / Wavelength: 0.9686 Å / Beamline: I24 / Wavelength: 0.9686 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Oct 7, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9686 Å / Relative weight: 1 |
| Reflection | Resolution: 3.099→43.116 Å / Num. obs: 33101 / % possible obs: 80.9 % / Redundancy: 3.5 % / Biso Wilson estimate: 61.74 Å2 / CC1/2: 0.998 / Rmerge(I) obs: 0.187 / Rpim(I) all: 0.153 / Rrim(I) all: 0.219 / Net I/σ(I): 6.6 |
| Reflection shell | Resolution: 3.099→3.232 Å / Redundancy: 3.7 % / Rmerge(I) obs: 0.606 / Mean I/σ(I) obs: 2.7 / Num. unique obs: 1654 / CC1/2: 0.792 / Rpim(I) all: 0.348 / Rrim(I) all: 0.702 / % possible all: 34.8 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4BW5 Resolution: 3.1→41.16 Å / Cor.coef. Fo:Fc: 0.801 / Cor.coef. Fo:Fc free: 0.826 / Cross valid method: THROUGHOUT / σ(F): 0 / SU Rfree Blow DPI: 0.419 Details: REFINED AGAINST STARANISO ANISOTROPICALLY TRUNCAED DATA. POSITIVE DIFFRERENCE PEAKS FOR WATER MOLECULES WERE SEEN, CORRESPONDING TO WATER POSITIONS IN 6RV2 and 6RV3, BUT WERE NOT MODELLED AT THIS RESOLUTION.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 48.81 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.54 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.1→41.16 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 3.1→3.15 Å / Total num. of bins used: 50
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj