[English] 日本語
 Yorodumi
Yorodumi- PDB-6qdj: Molecular features of the UNC-45 chaperone critical for binding a... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6qdj | ||||||
|---|---|---|---|---|---|---|---|
| Title | Molecular features of the UNC-45 chaperone critical for binding and folding muscle myosin | ||||||
 Components Components | Myosin-4 | ||||||
 Keywords Keywords | MOTOR PROTEIN / MYOSIN / MHC-B / UNC-54 | ||||||
| Function / homology |  Function and homology information Function and homology informationegg-laying behavior / striated muscle myosin thick filament / skeletal muscle myosin thick filament assembly / A band / muscle myosin complex / myosin filament / locomotion / myosin II complex / sarcomere organization / structural constituent of muscle ...egg-laying behavior / striated muscle myosin thick filament / skeletal muscle myosin thick filament assembly / A band / muscle myosin complex / myosin filament / locomotion / myosin II complex / sarcomere organization / structural constituent of muscle / microfilament motor activity / muscle contraction / actin filament binding / ATP binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.884 Å MOLECULAR REPLACEMENT / Resolution: 1.884 Å | ||||||
 Authors Authors | Meinhart, A. / Clausen, T. / Arnese, R. | ||||||
| Funding support |  Austria, 1items Austria, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2019 Journal: Nat Commun / Year: 2019Title: Molecular features of the UNC-45 chaperone critical for binding and folding muscle myosin. Authors: Hellerschmied, D. / Lehner, A. / Franicevic, N. / Arnese, R. / Johnson, C. / Vogel, A. / Meinhart, A. / Kurzbauer, R. / Deszcz, L. / Gazda, L. / Geeves, M. / Clausen, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6qdj.cif.gz 6qdj.cif.gz | 178.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6qdj.ent.gz pdb6qdj.ent.gz | 136.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6qdj.json.gz 6qdj.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qd/6qdj https://data.pdbj.org/pub/pdb/validation_reports/qd/6qdj ftp://data.pdbj.org/pub/pdb/validation_reports/qd/6qdj ftp://data.pdbj.org/pub/pdb/validation_reports/qd/6qdj | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6qdkC  6qdlC  6qdmC  5m05S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 90372.297 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Trichoplusia ni (cabbage looper) / References: UniProt: P02566 Trichoplusia ni (cabbage looper) / References: UniProt: P02566 |
|---|
-Non-polymers , 7 types, 253 molecules 

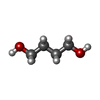
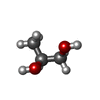

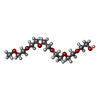







| #2: Chemical | ChemComp-ADP / | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| #3: Chemical | ChemComp-GOL / #4: Chemical | ChemComp-BU1 / | #5: Chemical | #6: Chemical | ChemComp-HEZ / | #7: Chemical | ChemComp-P15 / | #8: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.82 Å3/Da / Density % sol: 56.33 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 6.5 Details: 100 mM MES/imidazole pH 6.5 10 % PEG 4000 20 % glycerol 20 mM 1,6-hexandiol 20 mM 1-butanol 20 mM (RS)-1,2-propanediol 20 mM 2-propanol 20 mM 1,4-butanediol 20 mM 1,3-propandiol |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  PETRA III, EMBL c/o DESY PETRA III, EMBL c/o DESY  / Beamline: P13 (MX1) / Wavelength: 0.9763 Å / Beamline: P13 (MX1) / Wavelength: 0.9763 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Dec 17, 2016 |
| Radiation | Monochromator: KB-mirror / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9763 Å / Relative weight: 1 |
| Reflection | Resolution: 1.884→85 Å / Num. obs: 76208 / % possible obs: 93.8 % / Redundancy: 3.3 % / Biso Wilson estimate: 37.5 Å2 / CC1/2: 0.999 / Rrim(I) all: 0.045 / Net I/σ(I): 19.6 |
| Reflection shell | Resolution: 1.884→2.05 Å / Redundancy: 2.9 % / Mean I/σ(I) obs: 2.9 / CC1/2: 0.866 / Rrim(I) all: 0.454 / % possible all: 78 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5m05 Resolution: 1.884→84.026 Å / SU ML: 0.19 / Cross valid method: FREE R-VALUE / σ(F): 1.37 / Phase error: 22.81
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.884→84.026 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj














