[English] 日本語
 Yorodumi
Yorodumi- PDB-6oo5: Cryo-EM structure of the C2-symmetric TRPV2/RTx complex in amphip... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6oo5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the C2-symmetric TRPV2/RTx complex in amphipol resolved to 4.2 A | |||||||||
 Components Components | TRPV2 | |||||||||
 Keywords Keywords | METAL TRANSPORT / ion channel / calcium channel / TRP channel | |||||||||
| Function / homology |  Function and homology information Function and homology informationgrowth cone membrane / response to temperature stimulus / positive regulation of calcium ion import / calcium ion import across plasma membrane / positive regulation of axon extension / axonal growth cone / calcium channel activity / positive regulation of cold-induced thermogenesis / cell body / cell surface / identical protein binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 4.2 Å | |||||||||
 Authors Authors | Zubcevic, L. / Hsu, A.L. / Borgnia, M.J. / Lee, S.-Y. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2019 Journal: Elife / Year: 2019Title: Symmetry transitions during gating of the TRPV2 ion channel in lipid membranes. Authors: Lejla Zubcevic / Allen L Hsu / Mario J Borgnia / Seok-Yong Lee /  Abstract: The Transient Receptor Potential Vanilloid 2 (TRPV2) channel is a member of the temperature-sensing thermoTRPV family. Recent advances in cryo-electronmicroscopy (cryo-EM) and X-ray crystallography ...The Transient Receptor Potential Vanilloid 2 (TRPV2) channel is a member of the temperature-sensing thermoTRPV family. Recent advances in cryo-electronmicroscopy (cryo-EM) and X-ray crystallography have provided many important insights into the gating mechanisms of thermoTRPV channels. Interestingly, crystallographic studies of ligand-dependent TRPV2 gating have shown that the TRPV2 channel adopts two-fold symmetric arrangements during the gating cycle. However, it was unclear if crystal packing forces played a role in stabilizing the two-fold symmetric arrangement of the channel. Here, we employ cryo-EM to elucidate the structure of full-length rabbit TRPV2 in complex with the agonist resiniferatoxin (RTx) in nanodiscs and amphipol. We show that RTx induces two-fold symmetric conformations of TRPV2 in both environments. However, the two-fold symmetry is more pronounced in the native-like lipid environment of the nanodiscs. Our data offers insights into a gating pathway in TRPV2 involving symmetry transitions. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6oo5.cif.gz 6oo5.cif.gz | 412 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6oo5.ent.gz pdb6oo5.ent.gz | 324.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6oo5.json.gz 6oo5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6oo5_validation.pdf.gz 6oo5_validation.pdf.gz | 1.6 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6oo5_full_validation.pdf.gz 6oo5_full_validation.pdf.gz | 1.6 MB | Display | |
| Data in XML |  6oo5_validation.xml.gz 6oo5_validation.xml.gz | 73.7 KB | Display | |
| Data in CIF |  6oo5_validation.cif.gz 6oo5_validation.cif.gz | 111 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/oo/6oo5 https://data.pdbj.org/pub/pdb/validation_reports/oo/6oo5 ftp://data.pdbj.org/pub/pdb/validation_reports/oo/6oo5 ftp://data.pdbj.org/pub/pdb/validation_reports/oo/6oo5 | HTTPS FTP |
-Related structure data
| Related structure data |  20146MC  6oo3C  6oo4C  6oo7C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
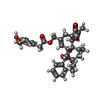
| #1: Protein | Mass: 88722.680 Da / Num. of mol.: 4 / Mutation: F470S, L505M, L508T, Q528E Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Chemical | ChemComp-6EU / |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: TRPV2 / Type: COMPLEX Details: TRPV2 in complex with RTx reconstituted into amphipol A8-35 Entity ID: #1 / Source: RECOMBINANT | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.300 MDa / Experimental value: NO | ||||||||||||||||||||
| Source (natural) | Organism:  | ||||||||||||||||||||
| Source (recombinant) | Organism:  | ||||||||||||||||||||
| Buffer solution | pH: 8 | ||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||
| Specimen | Conc.: 2 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: TRPV2 in complex with RTx reconstituted into amphipol A8-35, monodisperse | ||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil, UltrAuFoil, R1.2/1.3 | ||||||||||||||||||||
| Vitrification | Instrument: LEICA EM GP / Cryogen name: ETHANE / Humidity: 90 % / Chamber temperature: 296 K / Details: Blotted 3 seconds before plunging |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source: OTHER / Accelerating voltage: 300 kV / Illumination mode: OTHER |
| Electron lens | Mode: OTHER |
| Specimen holder | Cryogen: NITROGEN |
| Image recording | Electron dose: 42 e/Å2 / Film or detector model: FEI FALCON III (4k x 4k) / Num. of real images: 1293 |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.15_3459: / Classification: refinement | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| |||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | |||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C2 (2 fold cyclic) | |||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 4.2 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 90862 / Num. of class averages: 1 / Symmetry type: POINT | |||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT / Space: REAL | |||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 5AN8 Pdb chain-ID: A / Accession code: 5AN8 / Source name: PDB / Type: experimental model | |||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera












 PDBj
PDBj