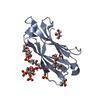
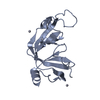
Entry Database : PDB / ID : 6jckTitle Complex structure of Axin-DIX and Dvl2-DIX Axin-1 Segment polarity protein dishevelled homolog DVL-2 Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 3.09 Å Authors Yamanishi, K. / Shibata, N. Funding support Organization Grant number Country Ministry of Education, Culture, Sports, Science and Technology (Japan) 23121526 Ministry of Education, Culture, Sports, Science and Technology (Japan) 25121731
Journal : Sci.Signal. / Year : 2019Title : A direct heterotypic interaction between the DIX domains of Dishevelled and Axin mediates signaling to beta-catenin.Authors : Yamanishi, K. / Fiedler, M. / Terawaki, S.I. / Higuchi, Y. / Bienz, M. / Shibata, N. History Deposition Jan 29, 2019 Deposition site / Processing site Revision 1.0 Jan 15, 2020 Provider / Type Revision 1.1 Nov 22, 2023 Group / Database references / Refinement descriptionCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model Item / _database_2.pdbx_database_accession
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.09 Å
MOLECULAR REPLACEMENT / Resolution: 3.09 Å  Authors
Authors Japan, 2items
Japan, 2items  Citation
Citation Journal: Sci.Signal. / Year: 2019
Journal: Sci.Signal. / Year: 2019 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6jck.cif.gz
6jck.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6jck.ent.gz
pdb6jck.ent.gz PDB format
PDB format 6jck.json.gz
6jck.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 6jck_validation.pdf.gz
6jck_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 6jck_full_validation.pdf.gz
6jck_full_validation.pdf.gz 6jck_validation.xml.gz
6jck_validation.xml.gz 6jck_validation.cif.gz
6jck_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/jc/6jck
https://data.pdbj.org/pub/pdb/validation_reports/jc/6jck ftp://data.pdbj.org/pub/pdb/validation_reports/jc/6jck
ftp://data.pdbj.org/pub/pdb/validation_reports/jc/6jck Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: AXIN1, AXIN / Plasmid: pCold-GST / Production host:
Homo sapiens (human) / Gene: AXIN1, AXIN / Plasmid: pCold-GST / Production host: 
 Homo sapiens (human) / Gene: DVL2 / Plasmid: pCold-GST / Production host:
Homo sapiens (human) / Gene: DVL2 / Plasmid: pCold-GST / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SPring-8
SPring-8  / Beamline: BL44XU / Wavelength: 0.9 Å
/ Beamline: BL44XU / Wavelength: 0.9 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller













 PDBj
PDBj










