+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6hw9 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Yeast 20S proteasome in complex with 41b | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE / Proteasome / Inhibitor / Binding Analysis | ||||||
| Function / homology |  Function and homology information Function and homology informationproteasome core complex assembly / nuclear outer membrane-endoplasmic reticulum membrane network / Proteasome assembly / Cross-presentation of soluble exogenous antigens (endosomes) / TNFR2 non-canonical NF-kB pathway / Ubiquitin-Mediated Degradation of Phosphorylated Cdc25A / proteasomal ubiquitin-independent protein catabolic process / Regulation of PTEN stability and activity / CDK-mediated phosphorylation and removal of Cdc6 / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis ...proteasome core complex assembly / nuclear outer membrane-endoplasmic reticulum membrane network / Proteasome assembly / Cross-presentation of soluble exogenous antigens (endosomes) / TNFR2 non-canonical NF-kB pathway / Ubiquitin-Mediated Degradation of Phosphorylated Cdc25A / proteasomal ubiquitin-independent protein catabolic process / Regulation of PTEN stability and activity / CDK-mediated phosphorylation and removal of Cdc6 / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / KEAP1-NFE2L2 pathway / Neddylation / Orc1 removal from chromatin / MAPK6/MAPK4 signaling / proteasome storage granule / Antigen processing: Ubiquitination & Proteasome degradation / proteasome endopeptidase complex / proteasome core complex, beta-subunit complex / endopeptidase activator activity / Ub-specific processing proteases / proteasome assembly / threonine-type endopeptidase activity / proteasome core complex, alpha-subunit complex / Neutrophil degranulation / proteasome complex / peroxisome / endopeptidase activity / proteasome-mediated ubiquitin-dependent protein catabolic process / mRNA binding / endoplasmic reticulum membrane / mitochondrion / nucleus / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.8 Å MOLECULAR REPLACEMENT / Resolution: 2.8 Å | ||||||
 Authors Authors | Huber, E.M. / Groll, M. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2019 Journal: J.Med.Chem. / Year: 2019Title: Structure-Based Design of Inhibitors Selective for Human Proteasome beta 2c or beta 2i Subunits. Authors: Xin, B.T. / Huber, E.M. / de Bruin, G. / Heinemeyer, W. / Maurits, E. / Espinal, C. / Du, Y. / Janssens, M. / Weyburne, E.S. / Kisselev, A.F. / Florea, B.I. / Driessen, C. / van der Marel, G. ...Authors: Xin, B.T. / Huber, E.M. / de Bruin, G. / Heinemeyer, W. / Maurits, E. / Espinal, C. / Du, Y. / Janssens, M. / Weyburne, E.S. / Kisselev, A.F. / Florea, B.I. / Driessen, C. / van der Marel, G.A. / Groll, M. / Overkleeft, H.S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6hw9.cif.gz 6hw9.cif.gz | 2.4 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6hw9.ent.gz pdb6hw9.ent.gz | 2 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6hw9.json.gz 6hw9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6hw9_validation.pdf.gz 6hw9_validation.pdf.gz | 1.9 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6hw9_full_validation.pdf.gz 6hw9_full_validation.pdf.gz | 1.9 MB | Display | |
| Data in XML |  6hw9_validation.xml.gz 6hw9_validation.xml.gz | 197.9 KB | Display | |
| Data in CIF |  6hw9_validation.cif.gz 6hw9_validation.cif.gz | 272.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hw/6hw9 https://data.pdbj.org/pub/pdb/validation_reports/hw/6hw9 ftp://data.pdbj.org/pub/pdb/validation_reports/hw/6hw9 ftp://data.pdbj.org/pub/pdb/validation_reports/hw/6hw9 | HTTPS FTP |
-Related structure data
| Related structure data |  6htbC  6htcC  6htdC  6htpC  6htrC  6hubC  6hucC  6huqC  6huuC  6huvC  6hv3C  6hv4C  6hv5C  6hv7C  6hvaC  6hvrC  6hvsC  6hvtC  6hvuC  6hvvC  6hvwC  6hvxC  6hvyC  6hw0C  6hw3C  6hw4C  6hw5C  6hw6C  6hw7C  6hw8C  6hwaC  6hwbC  6hwcC  6hwdC  6hweC  6hwfC  5cz4S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
NCS ensembles :
NCS oper:
|
- Components
Components
-Proteasome subunit alpha type- ... , 6 types, 12 molecules AOBPCQDRESGU
| #1: Protein | Mass: 27191.828 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P23639, proteasome endopeptidase complex #2: Protein | Mass: 28748.230 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P23638, proteasome endopeptidase complex #3: Protein | Mass: 28478.111 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P40303, proteasome endopeptidase complex #4: Protein | Mass: 28649.086 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P32379, proteasome endopeptidase complex #5: Protein | Mass: 25634.000 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P40302, proteasome endopeptidase complex #7: Protein | Mass: 28033.830 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21243, proteasome endopeptidase complex |
|---|
-Protein , 1 types, 2 molecules FT
| #6: Protein | Mass: 31575.068 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P21242, proteasome endopeptidase complex |
|---|
-Proteasome subunit beta type- ... , 7 types, 14 molecules HVIWJXKYLZMaNb
| #8: Protein | Mass: 25114.459 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P25043, proteasome endopeptidase complex #9: Protein | Mass: 22627.842 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P25451, proteasome endopeptidase complex #10: Protein | Mass: 22545.676 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P22141, proteasome endopeptidase complex #11: Protein | Mass: 23325.248 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P30656, proteasome endopeptidase complex #12: Protein | Mass: 24883.928 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P23724, proteasome endopeptidase complex #13: Protein | Mass: 27200.893 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P30657, proteasome endopeptidase complex #14: Protein | Mass: 21517.186 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  References: UniProt: P38624, proteasome endopeptidase complex |
|---|
-Non-polymers , 5 types, 318 molecules 

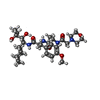






| #15: Chemical | ChemComp-MG / #16: Chemical | #17: Chemical | ChemComp-GWK / ( #18: Chemical | ChemComp-MES / #19: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.67 Å3/Da / Density % sol: 66.52 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 6.8 / Details: 20 mM MgAC2, 13% MPD, 0.1 M MES / PH range: 6.8 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06SA / Wavelength: 1 Å / Beamline: X06SA / Wavelength: 1 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Sep 7, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→50 Å / Num. obs: 257255 / % possible obs: 97.5 % / Redundancy: 3.1 % / Rmerge(I) obs: 0.074 / Net I/σ(I): 12.8 |
| Reflection shell | Resolution: 2.8→2.9 Å / Rmerge(I) obs: 0.579 / Mean I/σ(I) obs: 2.5 / % possible all: 97.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5CZ4 Resolution: 2.8→15 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.944 / SU B: 27.481 / SU ML: 0.221 / Cross valid method: THROUGHOUT / ESU R Free: 0.267 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 65.547 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.8→15 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller























 PDBj
PDBj
















