[English] 日本語
 Yorodumi
Yorodumi- PDB-6hr2: Crystal structure of PROTAC 2 in complex with the bromodomain of ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6hr2 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of PROTAC 2 in complex with the bromodomain of human SMARCA4 and pVHL:ElonginC:ElonginB | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSCRIPTION / bromodomain / BRG1 / SMARCA4 / PROTAC / VHL / ternary complex | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of glucose mediated signaling pathway / positive regulation of transcription of nucleolar large rRNA by RNA polymerase I / bBAF complex / regulation of cellular response to hypoxia / neural retina development / npBAF complex / nBAF complex / negative regulation of androgen receptor signaling pathway / EGR2 and SOX10-mediated initiation of Schwann cell myelination / nucleosome array spacer activity ...positive regulation of glucose mediated signaling pathway / positive regulation of transcription of nucleolar large rRNA by RNA polymerase I / bBAF complex / regulation of cellular response to hypoxia / neural retina development / npBAF complex / nBAF complex / negative regulation of androgen receptor signaling pathway / EGR2 and SOX10-mediated initiation of Schwann cell myelination / nucleosome array spacer activity / RHOBTB3 ATPase cycle / negative regulation of receptor signaling pathway via JAK-STAT / GBAF complex / regulation of G0 to G1 transition / Tat protein binding / transcription elongation factor activity / target-directed miRNA degradation / RNA polymerase I preinitiation complex assembly / elongin complex / RSC-type complex / ATP-dependent chromatin remodeler activity / regulation of nucleotide-excision repair / host-mediated activation of viral transcription / Replication of the SARS-CoV-1 genome / nucleosome disassembly / VCB complex / Cul5-RING ubiquitin ligase complex / SWI/SNF complex / regulation of mitotic metaphase/anaphase transition / Cul2-RING ubiquitin ligase complex / positive regulation of T cell differentiation / intracellular membraneless organelle / nuclear androgen receptor binding / positive regulation of double-strand break repair / SUMOylation of ubiquitinylation proteins / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / positive regulation of stem cell population maintenance / Regulation of MITF-M-dependent genes involved in pigmentation / regulation of G1/S transition of mitotic cell cycle / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / negative regulation of transcription elongation by RNA polymerase II / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / positive regulation of signal transduction by p53 class mediator / negative regulation of cell differentiation / positive regulation of Wnt signaling pathway / ATP-dependent activity, acting on DNA / positive regulation of myoblast differentiation / negative regulation of signal transduction / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / ubiquitin-like ligase-substrate adaptor activity / Formation of HIV elongation complex in the absence of HIV Tat / RNA Polymerase II Transcription Elongation / Chromatin modifying enzymes / Formation of RNA Pol II elongation complex / DNA polymerase binding / negative regulation of TORC1 signaling / RNA Polymerase II Pre-transcription Events / Interleukin-7 signaling / transcription initiation-coupled chromatin remodeling / negative regulation of autophagy / protein serine/threonine kinase binding / transcription corepressor binding / transcription coregulator binding / TP53 Regulates Transcription of DNA Repair Genes / transcription initiation at RNA polymerase II promoter / positive regulation of cell differentiation / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / transcription elongation by RNA polymerase II / Vif-mediated degradation of APOBEC3G / Formation of the beta-catenin:TCF transactivating complex / helicase activity / negative regulation of cell growth / Inactivation of CSF3 (G-CSF) signaling / Oxygen-dependent proline hydroxylation of Hypoxia-inducible Factor Alpha / Evasion by RSV of host interferon responses / kinetochore / positive regulation of miRNA transcription / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / Regulation of expression of SLITs and ROBOs / RMTs methylate histone arginines / nuclear matrix / fibrillar center / cell morphogenesis / ubiquitin-protein transferase activity / p53 binding / transcription corepressor activity / Antigen processing: Ubiquitination & Proteasome degradation / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / nervous system development / positive regulation of cold-induced thermogenesis / Neddylation / microtubule cytoskeleton / regulation of gene expression / protein-containing complex assembly / Replication of the SARS-CoV-2 genome / ubiquitin-dependent protein catabolic process / protein-macromolecule adaptor activity Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 1.76 Å SYNCHROTRON / Resolution: 1.76 Å | ||||||
 Authors Authors | Roy, M. / Bader, G. / Diers, E. / Trainor, N. / Farnaby, W. / Ciulli, A. | ||||||
 Citation Citation |  Journal: Nat.Chem.Biol. / Year: 2019 Journal: Nat.Chem.Biol. / Year: 2019Title: BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Authors: Farnaby, W. / Koegl, M. / Roy, M.J. / Whitworth, C. / Diers, E. / Trainor, N. / Zollman, D. / Steurer, S. / Karolyi-Oezguer, J. / Riedmueller, C. / Gmaschitz, T. / Wachter, J. / Dank, C. / ...Authors: Farnaby, W. / Koegl, M. / Roy, M.J. / Whitworth, C. / Diers, E. / Trainor, N. / Zollman, D. / Steurer, S. / Karolyi-Oezguer, J. / Riedmueller, C. / Gmaschitz, T. / Wachter, J. / Dank, C. / Galant, M. / Sharps, B. / Rumpel, K. / Traxler, E. / Gerstberger, T. / Schnitzer, R. / Petermann, O. / Greb, P. / Weinstabl, H. / Bader, G. / Zoephel, A. / Weiss-Puxbaum, A. / Ehrenhofer-Wolfer, K. / Wohrle, S. / Boehmelt, G. / Rinnenthal, J. / Arnhof, H. / Wiechens, N. / Wu, M.Y. / Owen-Hughes, T. / Ettmayer, P. / Pearson, M. / McConnell, D.B. / Ciulli, A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6hr2.cif.gz 6hr2.cif.gz | 762.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6hr2.ent.gz pdb6hr2.ent.gz | 639.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6hr2.json.gz 6hr2.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6hr2_validation.pdf.gz 6hr2_validation.pdf.gz | 1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6hr2_full_validation.pdf.gz 6hr2_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  6hr2_validation.xml.gz 6hr2_validation.xml.gz | 36.9 KB | Display | |
| Data in CIF |  6hr2_validation.cif.gz 6hr2_validation.cif.gz | 53.7 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hr/6hr2 https://data.pdbj.org/pub/pdb/validation_reports/hr/6hr2 ftp://data.pdbj.org/pub/pdb/validation_reports/hr/6hr2 ftp://data.pdbj.org/pub/pdb/validation_reports/hr/6hr2 | HTTPS FTP |
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 4 types, 8 molecules AEBFCGDH
| #1: Protein | Mass: 14155.381 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: SMARCA4, BAF190A, BRG1, SNF2B, SNF2L4 / Plasmid: pDEST15 / Production host: Homo sapiens (human) / Gene: SMARCA4, BAF190A, BRG1, SNF2B, SNF2L4 / Plasmid: pDEST15 / Production host:  References: UniProt: P51532, Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement #2: Protein | Mass: 17297.688 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: VHL / Plasmid: pHAT4 / Production host: Homo sapiens (human) / Gene: VHL / Plasmid: pHAT4 / Production host:  #3: Protein | Mass: 10974.616 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ELOC, TCEB1 / Plasmid: pCDF-1b DUET / Production host: Homo sapiens (human) / Gene: ELOC, TCEB1 / Plasmid: pCDF-1b DUET / Production host:  #4: Protein | Mass: 11748.406 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ELOB, TCEB2 / Plasmid: pCDF-1b DUET / Production host: Homo sapiens (human) / Gene: ELOB, TCEB2 / Plasmid: pCDF-1b DUET / Production host:  |
|---|
-Non-polymers , 4 types, 410 molecules 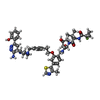






| #5: Chemical | | #6: Chemical | #7: Chemical | #8: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.66 Å3/Da / Density % sol: 53.69 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 6 / Details: 20% (w/v) PEG1500, 0.1 M MIB Buffer, pH 6.0 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06SA / Wavelength: 1 Å / Beamline: X06SA / Wavelength: 1 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: Aug 22, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 1.76→39.8 Å / Num. obs: 68052 / % possible obs: 89.4 % / Redundancy: 2.4 % / Biso Wilson estimate: 36.81 Å2 / Net I/σ(I): 12.1 |
| Reflection shell | Resolution: 1.76→1.96 Å |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.76→39.8 Å / Cor.coef. Fo:Fc: 0.941 / Cor.coef. Fo:Fc free: 0.935 / SU R Cruickshank DPI: 0.569 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.203 / SU Rfree Blow DPI: 0.162 / SU Rfree Cruickshank DPI: 0.166
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 279.02 Å2 / Biso mean: 56.7 Å2 / Biso min: 18.24 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.29 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.76→39.8 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.76→1.88 Å / Rfactor Rfree error: 0 / Total num. of bins used: 50
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj



















