[English] 日本語
 Yorodumi
Yorodumi- PDB-6h7n: ACTIVATED TURKEY BETA1 ADRENOCEPTOR WITH BOUND PARTIAL AGONIST XA... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6h7n | ||||||
|---|---|---|---|---|---|---|---|
| Title | ACTIVATED TURKEY BETA1 ADRENOCEPTOR WITH BOUND PARTIAL AGONIST XAMOTEROL AND NANOBODY Nb6B9 | ||||||
 Components Components |
| ||||||
 Keywords Keywords | IMMUNE SYSTEM / Beta1 Adrenoceptor / Activated / Partial Agonist / Nanobody | ||||||
| Function / homology |  Function and homology information Function and homology informationbeta1-adrenergic receptor activity / positive regulation of heart contraction / regulation of circadian sleep/wake cycle, sleep / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / DNA polymerase processivity factor activity / protein-disulfide reductase activity / adenylate cyclase-activating adrenergic receptor signaling pathway / cell redox homeostasis / early endosome / positive regulation of MAPK cascade ...beta1-adrenergic receptor activity / positive regulation of heart contraction / regulation of circadian sleep/wake cycle, sleep / norepinephrine-epinephrine-mediated vasodilation involved in regulation of systemic arterial blood pressure / DNA polymerase processivity factor activity / protein-disulfide reductase activity / adenylate cyclase-activating adrenergic receptor signaling pathway / cell redox homeostasis / early endosome / positive regulation of MAPK cascade / identical protein binding / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |    | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.5 Å MOLECULAR REPLACEMENT / Resolution: 2.5 Å | ||||||
 Authors Authors | Warne, T. / Edwards, P.C. / Dore, A.S. / Leslie, A.G.W. / Tate, C.G. | ||||||
 Citation Citation |  Journal: Biorxiv / Year: 2018 Journal: Biorxiv / Year: 2018Title: Molecular basis for high affinity agonist binding in GPCRs Authors: Warne, T. / Edwards, P.C. / Dore, A.S. / Leslie, A.G.W. / Tate, C.G. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6h7n.cif.gz 6h7n.cif.gz | 217 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6h7n.ent.gz pdb6h7n.ent.gz | 171.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6h7n.json.gz 6h7n.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/h7/6h7n https://data.pdbj.org/pub/pdb/validation_reports/h7/6h7n ftp://data.pdbj.org/pub/pdb/validation_reports/h7/6h7n ftp://data.pdbj.org/pub/pdb/validation_reports/h7/6h7n | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: _ / Refine code: _
NCS ensembles :
|
- Components
Components
-Protein , 2 types, 4 molecules EFAB
| #1: Protein | Mass: 11784.370 Da / Num. of mol.: 2 / Mutation: C32S,C35S Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Gene: trxA, fipA, tsnC, b3781, JW5856 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P0AA25 Trichoplusia ni (cabbage looper) / References: UniProt: P0AA25#2: Protein | Mass: 35002.777 Da / Num. of mol.: 2 / Mutation: R68S,M90V,C116L,F327A,F338M,C358A Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Trichoplusia ni (cabbage looper) / References: UniProt: P07700 Trichoplusia ni (cabbage looper) / References: UniProt: P07700 |
|---|
-Antibody , 1 types, 2 molecules CD
| #3: Antibody | Mass: 13036.453 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Non-polymers , 4 types, 34 molecules 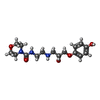

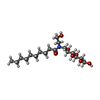




| #4: Chemical | | #5: Chemical | #6: Chemical | ChemComp-2CV / #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.85 Å3/Da / Density % sol: 68.05 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, sitting drop / pH: 7.5 / Details: 0.1 M Hepes-NaOH pH7.5 and 21-24% PEG1500 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-2 / Wavelength: 0.873 Å / Beamline: ID23-2 / Wavelength: 0.873 Å |
| Detector | Type: DECTRIS PILATUS3 2M / Detector: PIXEL / Date: Sep 25, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.873 Å / Relative weight: 1 |
| Reflection | Resolution: 2.5→41.5 Å / Num. obs: 64594 / % possible obs: 100 % / Redundancy: 11.9 % / Net I/σ(I): 5.4 |
| Reflection shell | Resolution: 2.5→2.56 Å |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3P0G, 2H6X Resolution: 2.5→41.5 Å / Cor.coef. Fo:Fc: 0.867 / Cor.coef. Fo:Fc free: 0.875 / SU B: 12.367 / SU ML: 0.264 / Cross valid method: THROUGHOUT / ESU R: 1.769 / ESU R Free: 0.401 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 60.682 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 2.5→41.5 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj













