+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ypw | ||||||
|---|---|---|---|---|---|---|---|
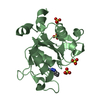
| Title | Crystal structure of IlvN.Val-1b | ||||||
 Components Components | Acetolactate synthase isozyme 1 small subunit | ||||||
 Keywords Keywords | TRANSFERASE / Transferase subunit / Regulatory subunit / ACT protein / amino acid binding | ||||||
| Function / homology |  Function and homology information Function and homology informationacetolactate synthase regulator activity / acetolactate synthase / acetolactate synthase activity / L-valine biosynthetic process / isoleucine biosynthetic process / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Sarma, S.P. / Bansal, A. / Schindelin, H. / Demeler, B. | ||||||
| Funding support |  India, 1items India, 1items
| ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2019 Journal: Biochemistry / Year: 2019Title: Crystallographic Structures of IlvN·Val/Ile Complexes: Conformational Selectivity for Feedback Inhibition of Aceto Hydroxy Acid Synthases. Authors: Bansal, A. / Karanth, N.M. / Demeler, B. / Schindelin, H. / Sarma, S.P. #1:  Journal: Biochemistry / Year: 2013 Journal: Biochemistry / Year: 2013Title: The coil-to-helix transition in IlvN regulates the allosteric control of Escherichia coli acetohydroxyacid synthase I. Authors: Karanth, N.M. / Sarma, S.P. #2: Journal: Biochemistry / Year: 2008 Title: Escherichia coli ilvN interacts with the FAD binding domain of ilvB and activates the AHAS I enzyme. Authors: Mitra, A. / Sarma, S.P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ypw.cif.gz 5ypw.cif.gz | 300.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ypw.ent.gz pdb5ypw.ent.gz | 245 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ypw.json.gz 5ypw.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yp/5ypw https://data.pdbj.org/pub/pdb/validation_reports/yp/5ypw ftp://data.pdbj.org/pub/pdb/validation_reports/yp/5ypw ftp://data.pdbj.org/pub/pdb/validation_reports/yp/5ypw | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5yppC  5ypyC  5yumC  2lvwS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Ens-ID: 1
|
 Movie
Movie Controller
Controller












 PDBj
PDBj
