+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5xmz | ||||||
|---|---|---|---|---|---|---|---|
| Title | Verticillium effector PevD1 | ||||||
 Components Components | Effector protein PevD1 | ||||||
 Keywords Keywords | SIGNALING PROTEIN / Verticillium / effector / PevD1 | ||||||
| Function / homology | : / Alternaria alternata allergen 1 / Alternaria alternata allergen 1 / Alt a 1 (AA1)-like domain profile. / extracellular region / Effector protein PevD1 Function and homology information Function and homology information | ||||||
| Biological species |  Verticillium dahliae (fungus) Verticillium dahliae (fungus) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SAD / Resolution: 1.85 Å SAD / Resolution: 1.85 Å | ||||||
 Authors Authors | Liu, X. / Zhou, R. | ||||||
 Citation Citation |  Journal: J. Exp. Bot. / Year: 2017 Journal: J. Exp. Bot. / Year: 2017Title: The asparagine-rich protein NRP interacts with the Verticillium effector PevD1 and regulates the subcellular localization of cryptochrome 2 Authors: Zhou, R. / Zhu, T. / Han, L. / Liu, M. / Xu, M. / Liu, Y. / Han, D. / Qiu, D. / Gong, Q. / Liu, X. | ||||||
| History |
|
- Structure visualization
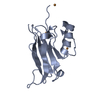
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5xmz.cif.gz 5xmz.cif.gz | 40.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5xmz.ent.gz pdb5xmz.ent.gz | 26.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5xmz.json.gz 5xmz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xm/5xmz https://data.pdbj.org/pub/pdb/validation_reports/xm/5xmz ftp://data.pdbj.org/pub/pdb/validation_reports/xm/5xmz ftp://data.pdbj.org/pub/pdb/validation_reports/xm/5xmz | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 16240.050 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Verticillium dahliae (fungus) / Production host: Verticillium dahliae (fungus) / Production host:  Enterobacteria phage L1 (virus) / References: UniProt: G0Y276 Enterobacteria phage L1 (virus) / References: UniProt: G0Y276 |
|---|---|
| #2: Chemical | ChemComp-CA / |
| #3: Chemical | ChemComp-CL / |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.97 Å3/Da / Density % sol: 37.56 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: batch mode / Details: 0.5M Sodium Formate, Tris pH7.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU MICROMAX-007 HF / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU MICROMAX-007 HF / Wavelength: 1.5418 Å |
| Detector | Type: MAR CCD 130 mm / Detector: CCD / Date: May 1, 2012 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.85→50 Å / Num. obs: 11198 / % possible obs: 98.4 % / Redundancy: 6.5 % / Rmerge(I) obs: 0.04 / Rsym value: 0.04 / Net I/σ(I): 15.3 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 1.85→35.805 Å / SU ML: 0.18 / Cross valid method: FREE R-VALUE / σ(F): 1.39 / Phase error: 25.68 SAD / Resolution: 1.85→35.805 Å / SU ML: 0.18 / Cross valid method: FREE R-VALUE / σ(F): 1.39 / Phase error: 25.68
| |||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.85→35.805 Å
| |||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj




