| Entry | Database: PDB / ID: 5vln
|
|---|
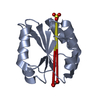
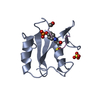
| Title | NMR structure of the N-domain of troponin C bound to switch region of troponin I |
|---|
 Components Components | Troponin C, slow skeletal and cardiac muscles,Troponin I, cardiac muscle |
|---|
 Keywords Keywords | METAL BINDING PROTEIN / cardiac troponin calcium binding protein EF hand / CELL INVASION |
|---|
| Function / homology |  Function and homology information Function and homology information
regulation of systemic arterial blood pressure by ischemic conditions / troponin C binding / diaphragm contraction / regulation of ATP-dependent activity / regulation of muscle filament sliding speed / troponin T binding / cardiac myofibril / cardiac Troponin complex / troponin complex / regulation of muscle contraction ...regulation of systemic arterial blood pressure by ischemic conditions / troponin C binding / diaphragm contraction / regulation of ATP-dependent activity / regulation of muscle filament sliding speed / troponin T binding / cardiac myofibril / cardiac Troponin complex / troponin complex / regulation of muscle contraction / regulation of smooth muscle contraction / negative regulation of ATP-dependent activity / transition between fast and slow fiber / Striated Muscle Contraction / muscle filament sliding / regulation of cardiac muscle contraction by calcium ion signaling / response to metal ion / ventricular cardiac muscle tissue morphogenesis / heart contraction / troponin I binding / vasculogenesis / skeletal muscle contraction / calcium channel inhibitor activity / cardiac muscle contraction / Ion homeostasis / sarcomere / intracellular calcium ion homeostasis / calcium-dependent protein binding / actin filament binding / heart development / actin binding / protein domain specific binding / calcium ion binding / protein kinase binding / protein homodimerization activity / cytosolSimilarity search - Function Troponin I residues 1-32 / Troponin I residues 1-32 / : / Troponin / Troponin domain superfamily / Troponin / EF-hand domain pair / : / EF-hand domain pair / EF-hand, calcium binding motif ...Troponin I residues 1-32 / Troponin I residues 1-32 / : / Troponin / Troponin domain superfamily / Troponin / EF-hand domain pair / : / EF-hand domain pair / EF-hand, calcium binding motif / EF-Hand 1, calcium-binding site / EF-hand calcium-binding domain. / EF-hand calcium-binding domain profile. / EF-hand domain / EF-hand domain pairSimilarity search - Domain/homology |
|---|
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
|---|
| Method | SOLUTION NMR / molecular dynamics |
|---|
 Authors Authors | Cai, F. / Hwang, P.M. / Sykes, B.D. |
|---|
| Funding support |  Canada, 1items Canada, 1items | Organization | Grant number | Country |
|---|
| Heart and Stroke Foundation of Canada | B.D.S., G-14-0005884 |  Canada Canada |
|
|---|
 Citation Citation |  Journal: J. Mol. Cell. Cardiol. / Year: 2016 Journal: J. Mol. Cell. Cardiol. / Year: 2016
Title: Structures reveal details of small molecule binding to cardiac troponin.
Authors: Cai, F. / Li, M.X. / Pineda-Sanabria, S.E. / Gelozia, S. / Lindert, S. / West, F. / Sykes, B.D. / Hwang, P.M. |
|---|
| History | | Deposition | Apr 25, 2017 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | May 24, 2017 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Jun 14, 2023 | Group: Data collection / Database references / Other
Category: database_2 / pdbx_database_status / pdbx_nmr_software
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_nmr_data / _pdbx_nmr_software.name |
|---|
| Revision 1.2 | May 15, 2024 | Group: Data collection / Database references / Category: chem_comp_atom / chem_comp_bond / database_2 / Item: _database_2.pdbx_DOI |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) Authors
Authors Canada, 1items
Canada, 1items  Citation
Citation Journal: J. Mol. Cell. Cardiol. / Year: 2016
Journal: J. Mol. Cell. Cardiol. / Year: 2016 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5vln.cif.gz
5vln.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5vln.ent.gz
pdb5vln.ent.gz PDB format
PDB format 5vln.json.gz
5vln.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/vl/5vln
https://data.pdbj.org/pub/pdb/validation_reports/vl/5vln ftp://data.pdbj.org/pub/pdb/validation_reports/vl/5vln
ftp://data.pdbj.org/pub/pdb/validation_reports/vl/5vln

 Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: TNNC1, TNNC, TNNI3, TNNC1 / Plasmid: pD444 / Production host:
Homo sapiens (human) / Gene: TNNC1, TNNC, TNNI3, TNNC1 / Plasmid: pD444 / Production host: 
 Sample preparation
Sample preparation Movie
Movie Controller
Controller











 PDBj
PDBj




 HSQC
HSQC