[English] 日本語
 Yorodumi
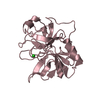
Yorodumi- PDB-5vah: Crystal structure of ATXR5 SET domain in complex with histone H3 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5vah | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of ATXR5 SET domain in complex with histone H3 di-methylated on R26 | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | TRANSFERASE/DNA BINDING PROTEIN / histone / nucleosome / methyltransferases / TRANSFERASE-DNA BINDING PROTEIN complex | |||||||||
| Function / homology |  Function and homology information Function and homology information[histone H3]-lysine27 N-methyltransferase / histone H3K27 monomethyltransferase activity / chromocenter / plastid / histone acetyltransferase activity / chloroplast / transcription coregulator activity / structural constituent of chromatin / nucleosome / methylation ...[histone H3]-lysine27 N-methyltransferase / histone H3K27 monomethyltransferase activity / chromocenter / plastid / histone acetyltransferase activity / chloroplast / transcription coregulator activity / structural constituent of chromatin / nucleosome / methylation / protein heterodimerization activity / chromatin binding / regulation of transcription by RNA polymerase II / chromatin / DNA binding / extracellular region / zinc ion binding / nucleus / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Ricinus communis (castor bean) Ricinus communis (castor bean) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.4 Å MOLECULAR REPLACEMENT / Resolution: 2.4 Å | |||||||||
 Authors Authors | Bergamin, E. / Sarvan, S. / Malette, J. / Eram, M. / Yeung, S. / Mongeon, V. / Joshi, M. / Brunzelle, J.S. / Michaels, S.D. / Blais, A. ...Bergamin, E. / Sarvan, S. / Malette, J. / Eram, M. / Yeung, S. / Mongeon, V. / Joshi, M. / Brunzelle, J.S. / Michaels, S.D. / Blais, A. / Vedadi, M. / Couture, J.-F. | |||||||||
 Citation Citation |  Journal: Nucleic Acids Res. / Year: 2017 Journal: Nucleic Acids Res. / Year: 2017Title: Molecular basis for the methylation specificity of ATXR5 for histone H3. Authors: Bergamin, E. / Sarvan, S. / Malette, J. / Eram, M.S. / Yeung, S. / Mongeon, V. / Joshi, M. / Brunzelle, J.S. / Michaels, S.D. / Blais, A. / Vedadi, M. / Couture, J.F. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5vah.cif.gz 5vah.cif.gz | 106.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5vah.ent.gz pdb5vah.ent.gz | 79.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5vah.json.gz 5vah.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/va/5vah https://data.pdbj.org/pub/pdb/validation_reports/va/5vah ftp://data.pdbj.org/pub/pdb/validation_reports/va/5vah ftp://data.pdbj.org/pub/pdb/validation_reports/va/5vah | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5va6C  5vabC  5vacC  5vbcC  4o30S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 26218.723 Da / Num. of mol.: 2 / Fragment: residues 146-374 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Ricinus communis (castor bean) / Gene: ATXR5, RCOM_1460410 / Production host: Ricinus communis (castor bean) / Gene: ATXR5, RCOM_1460410 / Production host:  References: UniProt: B9RU15, histone-lysine N-methyltransferase #2: Protein/peptide | Mass: 1415.637 Da / Num. of mol.: 2 / Fragment: residues 22-36 / Source method: obtained synthetically / Source: (synth.)  #3: Chemical | #4: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.52 Å3/Da / Density % sol: 51.2 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop Details: 50% polypropylene glycol 400, 5% DMSO, 0.1 M HEPES-NaOH (pH 6.0) |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 17-ID / Wavelength: 1 Å / Beamline: 17-ID / Wavelength: 1 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: May 16, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.4→17 Å / Num. obs: 19507 / % possible obs: 97.1 % / Redundancy: 3.5 % / Biso Wilson estimate: 51.13 Å2 / Rsym value: 0.061 / Net I/σ(I): 3.5 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4O30 Resolution: 2.4→16.93 Å / Cor.coef. Fo:Fc: 0.9112 / Cor.coef. Fo:Fc free: 0.8798 / SU R Cruickshank DPI: 0.47 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.475 / SU Rfree Blow DPI: 0.289 / SU Rfree Cruickshank DPI: 0.291
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 37.14 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.332 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 2.4→16.93 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.4→2.53 Å / Total num. of bins used: 10
|
 Movie
Movie Controller
Controller











 PDBj
PDBj




