[English] 日本語
 Yorodumi
Yorodumi- PDB-5v7q: Cryo-EM structure of the large ribosomal subunit from Mycobacteri... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5v7q | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the large ribosomal subunit from Mycobacterium tuberculosis bound with a potent linezolid analog | ||||||
 Components Components |
| ||||||
 Keywords Keywords | RIBOSOME / RNA dynamics | ||||||
| Function / homology |  Function and homology information Function and homology informationpeptidoglycan-based cell wall / large ribosomal subunit / transferase activity / 5S rRNA binding / ribosomal large subunit assembly / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation ...peptidoglycan-based cell wall / large ribosomal subunit / transferase activity / 5S rRNA binding / ribosomal large subunit assembly / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / mRNA binding / RNA binding / metal ion binding / plasma membrane / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.7 Å | ||||||
 Authors Authors | Yang, K. / Chang, J.-Y. / Cui, Z. / Zhang, J. | ||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2017 Journal: Nucleic Acids Res / Year: 2017Title: Structural insights into species-specific features of the ribosome from the human pathogen Mycobacterium tuberculosis. Authors: Kailu Yang / Jeng-Yih Chang / Zhicheng Cui / Xiaojun Li / Ran Meng / Lijun Duan / Jirapat Thongchol / Joanita Jakana / Christoph M Huwe / James C Sacchettini / Junjie Zhang /   Abstract: Ribosomes from Mycobacterium tuberculosis (Mtb) possess species-specific ribosomal RNA (rRNA) expansion segments and ribosomal proteins (rProtein). Here, we present the near-atomic structures of the ...Ribosomes from Mycobacterium tuberculosis (Mtb) possess species-specific ribosomal RNA (rRNA) expansion segments and ribosomal proteins (rProtein). Here, we present the near-atomic structures of the Mtb 50S ribosomal subunit and the complete Mtb 70S ribosome, solved by cryo-electron microscopy. Upon joining of the large and small ribosomal subunits, a 100-nt long expansion segment of the Mtb 23S rRNA, named H54a or the 'handle', switches interactions from with rRNA helix H68 and rProtein uL2 to with rProtein bS6, forming a new intersubunit bridge 'B9'. In Mtb 70S, bridge B9 is mostly maintained, leading to correlated motions among the handle, the L1 stalk and the small subunit in the rotated and non-rotated states. Two new protein densities were discovered near the decoding center and the peptidyl transferase center, respectively. These results provide a structural basis for studying translation in Mtb as well as developing new tuberculosis drugs. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5v7q.cif.gz 5v7q.cif.gz | 2 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5v7q.ent.gz pdb5v7q.ent.gz | 1.6 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5v7q.json.gz 5v7q.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/v7/5v7q https://data.pdbj.org/pub/pdb/validation_reports/v7/5v7q ftp://data.pdbj.org/pub/pdb/validation_reports/v7/5v7q ftp://data.pdbj.org/pub/pdb/validation_reports/v7/5v7q | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  8641MC  8645C  8646C  8647C  8648C  8649C  5v93C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
+50S ribosomal protein ... , 28 types, 28 molecules 012346CDEFGHJKLMNOPQSTUVWXYZ
-RNA chain , 2 types, 2 molecules AB
| #7: RNA chain | Mass: 1017792.562 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #8: RNA chain | Mass: 37097.238 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Protein / Non-polymers , 2 types, 2 molecules R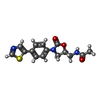

| #23: Protein | Mass: 11173.147 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #32: Chemical | ChemComp-917 / |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: RNA complex / Type: RIBOSOME / Entity ID: #1-#31 / Source: NATURAL |
|---|---|
| Source (natural) | Organism:  |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: JEOL 3200FSC |
|---|---|
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 2.37 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.11.1_2575: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: NONE | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 99285 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj






























