Entry Database : PDB / ID : 5qfzTitle PanDDA analysis group deposition -- Crystal structure of PTP1B in complex with compound_FMOPL000711a Tyrosine-protein phosphatase non-receptor type 1 Keywords / / / / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / / Resolution : 1.723 Å Authors Keedy, D.A. / Hill, Z.B. / Biel, J.T. / Kang, E. / Rettenmaier, T.J. / Brandao-Neto, J. / von Delft, F. / Wells, J.A. / Fraser, J.S. Journal : Elife / Year : 2018Title : An expanded allosteric network in PTP1B by multitemperature crystallography, fragment screening, and covalent tethering.Authors : Keedy, D.A. / Hill, Z.B. / Biel, J.T. / Kang, E. / Rettenmaier, T.J. / Brandao-Neto, J. / Pearce, N.M. / von Delft, F. / Wells, J.A. / Fraser, J.S. History Deposition Aug 30, 2018 Deposition site / Processing site Revision 1.0 Oct 10, 2018 Provider / Type Revision 1.1 Feb 6, 2019 Group / Database references / Structure summaryCategory / citation_author / entityItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year / _entity.formula_weight Revision 1.2 Nov 20, 2024 Group / Database references / Structure summaryCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature Item / _database_2.pdbx_database_accession
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.723 Å
molecular replacement / Resolution: 1.723 Å  Authors
Authors Citation
Citation Journal: Elife / Year: 2018
Journal: Elife / Year: 2018 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5qfz.cif.gz
5qfz.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5qfz.ent.gz
pdb5qfz.ent.gz PDB format
PDB format 5qfz.json.gz
5qfz.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/qf/5qfz
https://data.pdbj.org/pub/pdb/validation_reports/qf/5qfz ftp://data.pdbj.org/pub/pdb/validation_reports/qf/5qfz
ftp://data.pdbj.org/pub/pdb/validation_reports/qf/5qfz Links
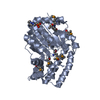
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: PTPN1, PTP1B / Plasmid: pET24b / Production host:
Homo sapiens (human) / Gene: PTPN1, PTP1B / Plasmid: pET24b / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  Diamond
Diamond  / Beamline: I04-1 / Wavelength: 0.92819 Å
/ Beamline: I04-1 / Wavelength: 0.92819 Å molecular replacement
molecular replacement Processing
Processing MOLECULAR REPLACEMENT / Resolution: 1.723→62.836 Å / SU ML: 0.23 / Cross valid method: THROUGHOUT / σ(F): 1.36 / Phase error: 20.96 / Stereochemistry target values: ML
MOLECULAR REPLACEMENT / Resolution: 1.723→62.836 Å / SU ML: 0.23 / Cross valid method: THROUGHOUT / σ(F): 1.36 / Phase error: 20.96 / Stereochemistry target values: ML Movie
Movie Controller
Controller





















 PDBj
PDBj





















