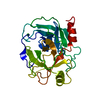
Entry Database : PDB / ID : 5ms4Title Kallikrein-related peptidase 8 leupeptin inhibitor complex Keywords / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)synthetic construct (others) Method / / / / Resolution : 2.1 Å Authors Debela, M. / Magdolen, V. / Skala, W. / Bode, W. / Brandstetter, H. / Goettig, P. Funding support Organization Grant number Country Austrian Science Fund P25003-B21
Journal : Sci Rep / Year : 2018Title : Structural determinants of specificity and regulation of activity in the allosteric loop network of human KLK8/neuropsin.Authors : Debela, M. / Magdolen, V. / Skala, W. / Elsasser, B. / Schneider, E.L. / Craik, C.S. / Biniossek, M.L. / Schilling, O. / Bode, W. / Brandstetter, H. / Goettig, P. History Deposition Dec 30, 2016 Deposition site / Processing site Revision 1.0 Jan 17, 2018 Provider / Type Revision 1.1 Aug 1, 2018 Group / Database references / Category / citation_authorItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year / _citation_author.name Revision 2.0 Sep 18, 2019 Group Advisory / Atomic model ... Advisory / Atomic model / Data collection / Database references / Derived calculations / Non-polymer description / Polymer sequence / Source and taxonomy / Structure summary Category atom_site / chem_comp ... atom_site / chem_comp / entity / entity_poly / entity_poly_seq / pdbx_distant_solvent_atoms / pdbx_entity_nonpoly / pdbx_entity_src_syn / pdbx_molecule_features / pdbx_nonpoly_scheme / pdbx_poly_seq_scheme / pdbx_struct_assembly / pdbx_struct_assembly_gen / pdbx_struct_conn_angle / pdbx_struct_sheet_hbond / pdbx_validate_close_contact / pdbx_validate_polymer_linkage / pdbx_validate_rmsd_bond / pdbx_validate_symm_contact / struct_asym / struct_conn / struct_ref / struct_ref_seq / struct_sheet / struct_sheet_order / struct_sheet_range / struct_site / struct_site_gen Item _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ... _atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_asym_id / _atom_site.auth_atom_id / _atom_site.auth_comp_id / _atom_site.auth_seq_id / _atom_site.group_PDB / _atom_site.label_asym_id / _atom_site.label_atom_id / _atom_site.label_comp_id / _atom_site.label_entity_id / _atom_site.label_seq_id / _atom_site.type_symbol / _chem_comp.formula / _chem_comp.formula_weight / _chem_comp.id / _chem_comp.mon_nstd_flag / _chem_comp.name / _chem_comp.pdbx_synonyms / _chem_comp.type / _entity.formula_weight / _entity.pdbx_description / _entity.type / _pdbx_distant_solvent_atoms.auth_asym_id / _pdbx_distant_solvent_atoms.auth_seq_id / _pdbx_distant_solvent_atoms.neighbor_ligand_distance / _pdbx_distant_solvent_atoms.neighbor_macromolecule_distance / _pdbx_struct_assembly.oligomeric_count / _pdbx_struct_assembly.oligomeric_details / _pdbx_struct_assembly_gen.asym_id_list / _pdbx_struct_conn_angle.ptnr2_auth_seq_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_validate_close_contact.auth_seq_id_1 / _pdbx_validate_close_contact.auth_seq_id_2 / _pdbx_validate_symm_contact.auth_seq_id_1 / _pdbx_validate_symm_contact.auth_seq_id_2 Revision 2.1 Oct 9, 2019 Group / Derived calculations / Category / struct_connRevision 2.2 Oct 9, 2024 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Structure summary Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature / struct_conn / struct_conn_type Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_conn.conn_type_id / _struct_conn.id / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_conn_type.id
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 2.1 Å
molecular replacement / Resolution: 2.1 Å  Authors
Authors Austria, 1items
Austria, 1items  Citation
Citation Journal: Sci Rep / Year: 2018
Journal: Sci Rep / Year: 2018 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5ms4.cif.gz
5ms4.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5ms4.ent.gz
pdb5ms4.ent.gz PDB format
PDB format 5ms4.json.gz
5ms4.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ms/5ms4
https://data.pdbj.org/pub/pdb/validation_reports/ms/5ms4 ftp://data.pdbj.org/pub/pdb/validation_reports/ms/5ms4
ftp://data.pdbj.org/pub/pdb/validation_reports/ms/5ms4 Links
Links Assembly
Assembly




 Components
Components Homo sapiens (human) / Gene: KLK8, NRPN, PRSS19, TADG14, UNQ283/PRO322 / Production host:
Homo sapiens (human) / Gene: KLK8, NRPN, PRSS19, TADG14, UNQ283/PRO322 / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SLS
SLS  / Beamline: X10SA / Wavelength: 1 Å
/ Beamline: X10SA / Wavelength: 1 Å molecular replacement
molecular replacement Processing
Processing MOLECULAR REPLACEMENT / Resolution: 2.1→65.53 Å / Cross valid method: FREE R-VALUE / σ(F): 2.38 / Phase error: 28.91
MOLECULAR REPLACEMENT / Resolution: 2.1→65.53 Å / Cross valid method: FREE R-VALUE / σ(F): 2.38 / Phase error: 28.91  Movie
Movie Controller
Controller





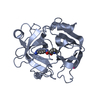





 PDBj
PDBj