[English] 日本語
 Yorodumi
Yorodumi- PDB-5lhp: The p-aminobenzamidine active site inhibited catalytic domain of ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5lhp | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | The p-aminobenzamidine active site inhibited catalytic domain of murine urokinase-type plasminogen activator in complex with the allosteric inhibitory nanobody Nb7 | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords | HYDROLASE / Trypsin-like serine proteases / Nanobody / Inhibitor | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationDissolution of Fibrin Clot / u-plasminogen activator / regulation of smooth muscle cell-matrix adhesion / urokinase plasminogen activator signaling pathway / regulation of plasminogen activation / regulation of fibrinolysis / protein complex involved in cell-matrix adhesion / negative regulation of plasminogen activation / serine-type endopeptidase complex / regulation of smooth muscle cell migration ...Dissolution of Fibrin Clot / u-plasminogen activator / regulation of smooth muscle cell-matrix adhesion / urokinase plasminogen activator signaling pathway / regulation of plasminogen activation / regulation of fibrinolysis / protein complex involved in cell-matrix adhesion / negative regulation of plasminogen activation / serine-type endopeptidase complex / regulation of smooth muscle cell migration / smooth muscle cell migration / plasminogen activation / regulation of cell adhesion mediated by integrin / negative regulation of fibrinolysis / regulation of cell adhesion / serine protease inhibitor complex / fibrinolysis / Neutrophil degranulation / regulation of cell population proliferation / response to hypoxia / positive regulation of cell migration / serine-type endopeptidase activity / external side of plasma membrane / extracellular space Similarity search - Function | |||||||||||||||
| Biological species |   | |||||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.63 Å MOLECULAR REPLACEMENT / Resolution: 2.63 Å | |||||||||||||||
 Authors Authors | Kromann-Hansen, T. / Lange, E.L. / Sorensen, H.P. / Ghassabeh, G.H. / Huang, M. / Jensen, J.K. / Muyldermans, S. / Declerck, P.J. / Andreasen, P.A. | |||||||||||||||
| Funding support |  Denmark, Denmark,  China, 4items China, 4items
| |||||||||||||||
 Citation Citation |  Journal: Sci Rep / Year: 2017 Journal: Sci Rep / Year: 2017Title: Discovery of a novel conformational equilibrium in urokinase-type plasminogen activator. Authors: Kromann-Hansen, T. / Louise Lange, E. / Peter Srensen, H. / Hassanzadeh-Ghassabeh, G. / Huang, M. / Jensen, J.K. / Muyldermans, S. / Declerck, P.J. / Komives, E.A. / Andreasen, P.A. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5lhp.cif.gz 5lhp.cif.gz | 92.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5lhp.ent.gz pdb5lhp.ent.gz | 69.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5lhp.json.gz 5lhp.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/lh/5lhp https://data.pdbj.org/pub/pdb/validation_reports/lh/5lhp ftp://data.pdbj.org/pub/pdb/validation_reports/lh/5lhp ftp://data.pdbj.org/pub/pdb/validation_reports/lh/5lhp | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5lhnC  5lhqC  5lhrSC  5lhsC  4jvpS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / Antibody , 2 types, 2 molecules AB
| #1: Protein | Mass: 27554.158 Da / Num. of mol.: 1 / Mutation: C122A Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #2: Antibody | Mass: 16303.926 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
-Non-polymers , 4 types, 101 molecules 

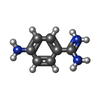




| #3: Chemical | ChemComp-SO4 / #4: Chemical | #5: Chemical | ChemComp-PBZ / | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.56 Å3/Da / Density % sol: 65.43 % |
|---|---|
| Crystal grow | Temperature: 288 K / Method: vapor diffusion, hanging drop / pH: 7.4 / Details: 100 mM HEPES, 1.6 M Li2SO4 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  MAX II MAX II  / Beamline: I911-2 / Wavelength: 1.04 Å / Beamline: I911-2 / Wavelength: 1.04 Å |
| Detector | Type: MAR CCD 165 mm / Detector: CCD / Date: Oct 1, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.04 Å / Relative weight: 1 |
| Reflection | Resolution: 2.63→40.738 Å / Num. obs: 18943 / % possible obs: 99.2 % / Redundancy: 6.9 % / Rmerge(I) obs: 0.088 / Net I/σ(I): 22.94 |
| Reflection shell | Resolution: 2.63→2.77 Å / Rmerge(I) obs: 1.017 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5LHR, 4JVP Resolution: 2.63→40.738 Å / SU ML: 0.35 / Cross valid method: FREE R-VALUE / σ(F): 1.36 / Phase error: 25.87 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.63→40.738 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj















