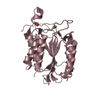
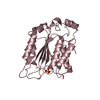
Entry Database : PDB / ID : 5jzvTitle The structure of D77G hCINAP-ADP Adenylate kinase isoenzyme 6 Keywords / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / Resolution : 2.07 Å Authors Liu, Y. / Yang, Z. / Yang, Y. / Cai, X. / Zheng, X. Funding support Organization Grant number Country National Science Foundation of China 31470754 Doctoral Fund of the Ministry of Education of China 20130001130003 Beijing Natural Science Foundation Grant 5152012
Journal : Nat Commun / Year : 2016Title : The ATPase hCINAP regulates 18S rRNA processing and is essential for embryogenesis and tumour growth.Authors : Bai, D. / Zhang, J. / Li, T. / Hang, R. / Liu, Y. / Tian, Y. / Huang, D. / Qu, L. / Cao, X. / Ji, J. / Zheng, X. History Deposition May 17, 2016 Deposition site / Processing site Revision 1.0 Aug 10, 2016 Provider / Type Revision 1.1 Dec 6, 2017 Group / Derived calculationsCategory / citation_author / pdbx_struct_oper_listItem _citation.journal_volume / _citation.page_first ... _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _pdbx_struct_oper_list.symmetry_operation Revision 1.2 Nov 8, 2023 Group / Database references / Refinement descriptionCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model Item / _database_2.pdbx_database_accession
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.07 Å
MOLECULAR REPLACEMENT / Resolution: 2.07 Å  Authors
Authors China, 3items
China, 3items  Citation
Citation Journal: Nat Commun / Year: 2016
Journal: Nat Commun / Year: 2016 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5jzv.cif.gz
5jzv.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5jzv.ent.gz
pdb5jzv.ent.gz PDB format
PDB format 5jzv.json.gz
5jzv.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/jz/5jzv
https://data.pdbj.org/pub/pdb/validation_reports/jz/5jzv ftp://data.pdbj.org/pub/pdb/validation_reports/jz/5jzv
ftp://data.pdbj.org/pub/pdb/validation_reports/jz/5jzv
 Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: AK6, CINAP, AD-004, CGI-137 / Plasmid: pET28a / Production host:
Homo sapiens (human) / Gene: AK6, CINAP, AD-004, CGI-137 / Plasmid: pET28a / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site: BSRF
SYNCHROTRON / Site: BSRF  / Beamline: 3W1A / Wavelength: 0.9792 Å
/ Beamline: 3W1A / Wavelength: 0.9792 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller











 PDBj
PDBj



