[English] 日本語
 Yorodumi
Yorodumi- PDB-5grb: Crystal structure of 2C helicase from enterovirus 71 (EV71) bound... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5grb | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of 2C helicase from enterovirus 71 (EV71) bound with ATPgammaS | ||||||
 Components Components | EV71 2C ATPase | ||||||
 Keywords Keywords | HYDROLASE/INHIBITOR / Enterovirus 2C ATPase / virus replication / Zinc finger / AAA+ ATPase / HYDROLASE-INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase ...symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / clathrin-dependent endocytosis of virus by host cell / DNA replication / RNA helicase activity / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / ATP hydrolysis activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | ||||||
| Biological species |  Enterovirus Enterovirus | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.803 Å MOLECULAR REPLACEMENT / Resolution: 2.803 Å | ||||||
 Authors Authors | Guan, H.X. / Tian, J. / Qin, B. / Wojdyla, J. / Wang, M.T. / Cui, S. | ||||||
| Funding support |  China, 1items China, 1items
| ||||||
 Citation Citation |  Journal: SCI ADV / Year: 2017 Journal: SCI ADV / Year: 2017Title: Crystal structure of 2C helicase from enterovirus 71 Authors: Guan, H.X. / Tian, J. / Qin, B. / Wojdyla, J. / Wang, B. / Zhao, Z.D. / Wang, M.T. / Cui, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5grb.cif.gz 5grb.cif.gz | 252.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5grb.ent.gz pdb5grb.ent.gz | 204.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5grb.json.gz 5grb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5grb_validation.pdf.gz 5grb_validation.pdf.gz | 2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5grb_full_validation.pdf.gz 5grb_full_validation.pdf.gz | 2.1 MB | Display | |
| Data in XML |  5grb_validation.xml.gz 5grb_validation.xml.gz | 52.6 KB | Display | |
| Data in CIF |  5grb_validation.cif.gz 5grb_validation.cif.gz | 69.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gr/5grb https://data.pdbj.org/pub/pdb/validation_reports/gr/5grb ftp://data.pdbj.org/pub/pdb/validation_reports/gr/5grb ftp://data.pdbj.org/pub/pdb/validation_reports/gr/5grb | HTTPS FTP |
-Related structure data
| Related structure data |  5gq1SC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 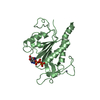
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 23541.812 Da / Num. of mol.: 6 / Mutation: E207A, K209A Source method: isolated from a genetically manipulated source Details: EV71 2C variant lacks N-terminal 1-115aa and contains mutation E207A, K209A. Source: (gene. exp.)  Enterovirus / Production host: Enterovirus / Production host:  #2: Chemical | ChemComp-AGS / #3: Chemical | ChemComp-ZN / #4: Water | ChemComp-HOH / | Sequence details | AUTHORS STATE THAT THE GENEBANK ACCESSION NUMBER IS ACM62759.1 FOR THIS SEQUENCE. THERE ARE ...AUTHORS STATE THAT THE GENEBANK ACCESSION NUMBER IS ACM62759.1 FOR THIS SEQUENCE. THERE ARE ENGINEERED | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.29 Å3/Da / Density % sol: 46.29 % Description: THE ENTRY CONTAINS FRIEDEL PAIRS IN F_PLUS/MINUS COLUMNS. |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, hanging drop / pH: 7.5 Details: 0.1M HEPES, 10% PEG6000, 5% MPD and 0.5% w/v polyvinylpyrrolidone K15 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRF SSRF  / Beamline: BL18U1 / Wavelength: 0.97776 Å / Beamline: BL18U1 / Wavelength: 0.97776 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Jun 12, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97776 Å / Relative weight: 1 |
| Reflection | Resolution: 2.8→45.548 Å / Num. obs: 59800 / % possible obs: 95.3 % / Observed criterion σ(I): -3 / Redundancy: 2.26 % / Biso Wilson estimate: 76.169 Å2 / CC1/2: 0.998 / Rsym value: 0.053 / Net I/σ(I): 10.88 |
| Reflection shell | Resolution: 2.8→2.97 Å / Redundancy: 1.85 % / Rmerge(I) obs: 0.7 / Mean I/σ(I) obs: 1.06 / CC1/2: 0.583 / % possible all: 88.8 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5GQ1 Resolution: 2.803→45.548 Å / SU ML: 0.56 / Cross valid method: THROUGHOUT / σ(F): 1.34 / Phase error: 34.14 / Stereochemistry target values: ML Details: SF FILE CONTAINS FRIEDEL PAIRS UNDER I/F_MINUS AND I/F_PLUS COLUMNS.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.803→45.548 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj








