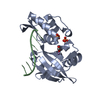
Entry Database : PDB / ID : 5gnvTitle Structure of PSD-95/MAP1A complex reveals unique target recognition mode of MAGUK GK domain Disks large homolog 4 Microtubule-associated protein 1A Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Rattus norvegicus (Norway rat)Mus musculus (house mouse)Method / / / Resolution : 2.596 Å Authors Shang, Y. / Xia, Y. / Zhu, R. / Zhu, J. Funding support Organization Grant number Country National Natural Science Foundation of China 31470733 the Shanghai YangFan Plan for Young Scientists 14YF1406700 the SIBS Frontier Science Program for talented young scientists 2014KIP101
Journal : Biochem. J. / Year : 2017Title : Structure of the PSD-95/MAP1A complex reveals a unique target recognition mode of the MAGUK GK domainAuthors : Xia, Y. / Shang, Y. / Zhang, R. / Zhu, J. History Deposition Jul 25, 2016 Deposition site / Processing site Revision 1.0 Aug 2, 2017 Provider / Type Revision 1.1 Nov 1, 2017 Group / Category / citation_authorItem _citation.country / _citation.journal_abbrev ... _citation.country / _citation.journal_abbrev / _citation.journal_id_ASTM / _citation.journal_id_CSD / _citation.journal_id_ISSN / _citation.journal_volume / _citation.page_first / _citation.page_last / _citation.pdbx_database_id_DOI / _citation.pdbx_database_id_PubMed / _citation.title / _citation.year / _citation_author.name Revision 1.2 Mar 20, 2024 Group / Database references / Derived calculationsCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_struct_special_symmetry Item / _database_2.pdbx_database_accession
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information

 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.596 Å
MOLECULAR REPLACEMENT / Resolution: 2.596 Å  Authors
Authors China, 3items
China, 3items  Citation
Citation Journal: Biochem. J. / Year: 2017
Journal: Biochem. J. / Year: 2017 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5gnv.cif.gz
5gnv.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5gnv.ent.gz
pdb5gnv.ent.gz PDB format
PDB format 5gnv.json.gz
5gnv.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 5gnv_validation.pdf.gz
5gnv_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 5gnv_full_validation.pdf.gz
5gnv_full_validation.pdf.gz 5gnv_validation.xml.gz
5gnv_validation.xml.gz 5gnv_validation.cif.gz
5gnv_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/gn/5gnv
https://data.pdbj.org/pub/pdb/validation_reports/gn/5gnv ftp://data.pdbj.org/pub/pdb/validation_reports/gn/5gnv
ftp://data.pdbj.org/pub/pdb/validation_reports/gn/5gnv Links
Links Assembly
Assembly
 Components
Components


 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SSRF
SSRF  / Beamline: BL17U / Wavelength: 0.97928 Å
/ Beamline: BL17U / Wavelength: 0.97928 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 2.596→40.089 Å / SU ML: 0.33 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 28.49 / Stereochemistry target values: ML
MOLECULAR REPLACEMENT / Resolution: 2.596→40.089 Å / SU ML: 0.33 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 28.49 / Stereochemistry target values: ML Movie
Movie Controller
Controller











 PDBj
PDBj






