+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: PDB / ID: 5flc | ||||||
|---|---|---|---|---|---|---|---|
| タイトル | Architecture of human mTOR Complex 1 - 5.9 Angstrom reconstruction | ||||||
 要素 要素 |
| ||||||
 キーワード キーワード | TRANSFERASE / RAPAMYCIN / MTORC1 | ||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報RNA polymerase III type 2 promoter sequence-specific DNA binding / RNA polymerase III type 1 promoter sequence-specific DNA binding / positive regulation of cytoplasmic translational initiation / regulation of locomotor rhythm / T-helper 1 cell lineage commitment / positive regulation of pentose-phosphate shunt / positive regulation of wound healing, spreading of epidermal cells / TORC2 signaling / TORC2 complex / regulation of membrane permeability ...RNA polymerase III type 2 promoter sequence-specific DNA binding / RNA polymerase III type 1 promoter sequence-specific DNA binding / positive regulation of cytoplasmic translational initiation / regulation of locomotor rhythm / T-helper 1 cell lineage commitment / positive regulation of pentose-phosphate shunt / positive regulation of wound healing, spreading of epidermal cells / TORC2 signaling / TORC2 complex / regulation of membrane permeability / cellular response to leucine starvation / heart valve morphogenesis / TFIIIC-class transcription factor complex binding / negative regulation of lysosome organization / TORC1 complex / voluntary musculoskeletal movement / positive regulation of transcription of nucleolar large rRNA by RNA polymerase I / calcineurin-NFAT signaling cascade / RNA polymerase III type 3 promoter sequence-specific DNA binding / positive regulation of keratinocyte migration / regulation of osteoclast differentiation / regulation of lysosome organization / MTOR signalling / cellular response to L-leucine / energy reserve metabolic process / regulation of autophagosome assembly / Energy dependent regulation of mTOR by LKB1-AMPK / cellular response to nutrient / Amino acids regulate mTORC1 / TORC1 signaling / cellular response to methionine / serine/threonine protein kinase complex / ruffle organization / negative regulation of cell size / positive regulation of ubiquitin-dependent protein catabolic process / cellular response to osmotic stress / anoikis / negative regulation of protein localization to nucleus / inositol hexakisphosphate binding / cardiac muscle cell development / negative regulation of calcineurin-NFAT signaling cascade / regulation of myelination / positive regulation of transcription by RNA polymerase III / negative regulation of macroautophagy / Macroautophagy / positive regulation of myotube differentiation / regulation of cell size / Constitutive Signaling by AKT1 E17K in Cancer / positive regulation of actin filament polymerization / germ cell development / behavioral response to pain / oligodendrocyte differentiation / positive regulation of oligodendrocyte differentiation / TOR signaling / positive regulation of translational initiation / mTORC1-mediated signalling / CD28 dependent PI3K/Akt signaling / HSF1-dependent transactivation / regulation of macroautophagy / positive regulation of TOR signaling / 'de novo' pyrimidine nucleobase biosynthetic process / response to amino acid / positive regulation of epithelial to mesenchymal transition / vascular endothelial cell response to laminar fluid shear stress / positive regulation of lipid biosynthetic process / heart morphogenesis / cellular response to nutrient levels / neuronal action potential / regulation of cellular response to heat / positive regulation of lamellipodium assembly / cardiac muscle contraction / positive regulation of stress fiber assembly / T cell costimulation / phagocytic vesicle / endomembrane system / cytoskeleton organization / negative regulation of insulin receptor signaling pathway / negative regulation of autophagy / cellular response to amino acid starvation / positive regulation of glycolytic process / cellular response to starvation / regulation of signal transduction by p53 class mediator / Regulation of PTEN gene transcription / protein serine/threonine kinase activator activity / positive regulation of translation / VEGFR2 mediated vascular permeability / post-embryonic development / TP53 Regulates Metabolic Genes / regulation of actin cytoskeleton organization / non-specific protein-tyrosine kinase / cellular response to amino acid stimulus / macroautophagy / regulation of cell growth / response to nutrient levels / phosphoprotein binding / regulation of circadian rhythm / PML body / protein destabilization / response to virus / multicellular organism growth 類似検索 - 分子機能 | ||||||
| 生物種 |  HOMO SAPIENS (ヒト) HOMO SAPIENS (ヒト) | ||||||
| 手法 | 電子顕微鏡法 / 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 5.9 Å | ||||||
 データ登録者 データ登録者 | Aylett, C.H.S. / Sauer, E. / Imseng, S. / Boehringer, D. / Hall, M.N. / Ban, N. / Maier, T. | ||||||
 引用 引用 |  ジャーナル: Science / 年: 2016 ジャーナル: Science / 年: 2016タイトル: Architecture of human mTOR complex 1. 著者: Christopher H S Aylett / Evelyn Sauer / Stefan Imseng / Daniel Boehringer / Michael N Hall / Nenad Ban / Timm Maier /  要旨: Target of rapamycin (TOR), a conserved protein kinase and central controller of cell growth, functions in two structurally and functionally distinct complexes: TORC1 and TORC2. Dysregulation of ...Target of rapamycin (TOR), a conserved protein kinase and central controller of cell growth, functions in two structurally and functionally distinct complexes: TORC1 and TORC2. Dysregulation of mammalian TOR (mTOR) signaling is implicated in pathologies that include diabetes, cancer, and neurodegeneration. We resolved the architecture of human mTORC1 (mTOR with subunits Raptor and mLST8) bound to FK506 binding protein (FKBP)-rapamycin, by combining cryo-electron microscopy at 5.9 angstrom resolution with crystallographic studies of Chaetomium thermophilum Raptor at 4.3 angstrom resolution. The structure explains how FKBP-rapamycin and architectural elements of mTORC1 limit access to the recessed active site. Consistent with a role in substrate recognition and delivery, the conserved amino-terminal domain of Raptor is juxtaposed to the kinase active site. | ||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | 分子:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- ダウンロードとリンク
ダウンロードとリンク
- ダウンロード
ダウンロード
| PDBx/mmCIF形式 |  5flc.cif.gz 5flc.cif.gz | 1016.8 KB | 表示 |  PDBx/mmCIF形式 PDBx/mmCIF形式 |
|---|---|---|---|---|
| PDB形式 |  pdb5flc.ent.gz pdb5flc.ent.gz | 817.7 KB | 表示 |  PDB形式 PDB形式 |
| PDBx/mmJSON形式 |  5flc.json.gz 5flc.json.gz | ツリー表示 |  PDBx/mmJSON形式 PDBx/mmJSON形式 | |
| その他 |  その他のダウンロード その他のダウンロード |
-検証レポート
| 文書・要旨 |  5flc_validation.pdf.gz 5flc_validation.pdf.gz | 1.1 MB | 表示 |  wwPDB検証レポート wwPDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  5flc_full_validation.pdf.gz 5flc_full_validation.pdf.gz | 1.3 MB | 表示 | |
| XML形式データ |  5flc_validation.xml.gz 5flc_validation.xml.gz | 137.1 KB | 表示 | |
| CIF形式データ |  5flc_validation.cif.gz 5flc_validation.cif.gz | 230.1 KB | 表示 | |
| アーカイブディレクトリ |  https://data.pdbj.org/pub/pdb/validation_reports/fl/5flc https://data.pdbj.org/pub/pdb/validation_reports/fl/5flc ftp://data.pdbj.org/pub/pdb/validation_reports/fl/5flc ftp://data.pdbj.org/pub/pdb/validation_reports/fl/5flc | HTTPS FTP |
-関連構造データ
- リンク
リンク
- 集合体
集合体
| 登録構造単位 | 
|
|---|---|
| 1 |
|
- 要素
要素
-SERINE/THREONINE-PROTEIN KINASE ... , 3種, 6分子 1324BF
| #1: タンパク質 | 分子量: 52357.672 Da / 分子数: 2 / 断片: HORN DOMAIN / 由来タイプ: 組換発現 / 由来: (組換発現)  HOMO SAPIENS (ヒト) / 細胞株 (発現宿主): Sf21 HOMO SAPIENS (ヒト) / 細胞株 (発現宿主): Sf21発現宿主:  参照: non-specific serine/threonine protein kinase #2: タンパク質 | 分子量: 31081.197 Da / 分子数: 2 / 断片: BRIDGE DOMAIN / 由来タイプ: 組換発現 / 由来: (組換発現)  HOMO SAPIENS (ヒト) / 細胞株 (発現宿主): Sf21 HOMO SAPIENS (ヒト) / 細胞株 (発現宿主): Sf21発現宿主:  参照: non-specific serine/threonine protein kinase #4: タンパク質 | 分子量: 134036.641 Da / 分子数: 2 / 断片: FAT AND PIKK DOMAINS / 由来タイプ: 組換発現 / 由来: (組換発現)  HOMO SAPIENS (ヒト) / 細胞株 (発現宿主): Sf21 HOMO SAPIENS (ヒト) / 細胞株 (発現宿主): Sf21発現宿主:  参照: UniProt: P42345, non-specific serine/threonine protein kinase |
|---|
-タンパク質 , 3種, 6分子 AECGDH
| #3: タンパク質 | 分子量: 87589.977 Da / 分子数: 2 / 由来タイプ: 組換発現 / 由来: (組換発現)  HOMO SAPIENS (ヒト) / 細胞株 (発現宿主): Sf21 HOMO SAPIENS (ヒト) / 細胞株 (発現宿主): Sf21発現宿主:  #5: タンパク質 | 分子量: 9124.238 Da / 分子数: 2 / 由来タイプ: 天然 由来: (天然)  #6: タンパク質 | 分子量: 35910.090 Da / 分子数: 2 / 由来タイプ: 組換発現 / 由来: (組換発現)  HOMO SAPIENS (ヒト) / 細胞株 (発現宿主): Sf21 HOMO SAPIENS (ヒト) / 細胞株 (発現宿主): Sf21発現宿主:  参照: UniProt: Q9BVC4 |
|---|
-非ポリマー , 1種, 2分子 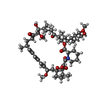
| #7: 化合物 |
|---|
-実験情報
-実験
| 実験 | 手法: 電子顕微鏡法 |
|---|---|
| EM実験 | 試料の集合状態: PARTICLE / 3次元再構成法: 単粒子再構成法 |
- 試料調製
試料調製
| 構成要素 | 名称: HUMAN MTOR COMPLEX 1 / タイプ: COMPLEX |
|---|---|
| 緩衝液 | 名称: 100 MM NACL, 10 MM NABICINE, 1 MM TCEP / pH: 8 / 詳細: 100 MM NACL, 10 MM NABICINE, 1 MM TCEP |
| 試料 | 包埋: NO / シャドウイング: NO / 染色: NO / 凍結: YES |
| 試料支持 | 詳細: CARBON |
| 急速凍結 | 装置: FEI VITROBOT MARK I / 凍結剤: ETHANE 詳細: VITRIFICATION 1 -- CRYOGEN- ETHANE, HUMIDITY- 100, TEMPERATURE- 120, INSTRUMENT- FEI VITROBOT MARK I, METHOD- 4 SECOND BLOTTING, |
- 電子顕微鏡撮影
電子顕微鏡撮影
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
|---|---|
| 顕微鏡 | モデル: FEI TITAN KRIOS / 日付: 2015年5月5日 |
| 電子銃 | 電子線源:  FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM FIELD EMISSION GUN / 加速電圧: 300 kV / 照射モード: FLOOD BEAM |
| 電子レンズ | モード: BRIGHT FIELD / 倍率(公称値): 59000 X / 倍率(補正後): 100719 X / 最大 デフォーカス(公称値): 4000 nm / 最小 デフォーカス(公称値): 1900 nm / Cs: 2.7 mm |
| 試料ホルダ | 温度: 100 K |
| 撮影 | 電子線照射量: 25 e/Å2 フィルム・検出器のモデル: FEI FALCON II (4k x 4k) |
- 解析
解析
| EMソフトウェア |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF補正 | 詳細: EACH IMAGE | ||||||||||||||||
| 対称性 | 点対称性: C2 (2回回転対称) | ||||||||||||||||
| 3次元再構成 | 手法: MAXIMUM A POSTERIORI PROJECTION MATCHING / 解像度: 5.9 Å / 粒子像の数: 309792 / ピクセルサイズ(公称値): 1.39 Å / ピクセルサイズ(実測値): 1.39 Å 詳細: THE UNK CHAINS (A, E, C, G, 1-4) CORRESPONDING TO RAPTOR (A,E), SF FKBP AND RAPAMYCIN (C,G) AND THE N-TERMINAL HEAT REPEATS OF MTOR (1-4), HAVE BEEN NUMBERED FROM 100 AT EACH BREAK TO ...詳細: THE UNK CHAINS (A, E, C, G, 1-4) CORRESPONDING TO RAPTOR (A,E), SF FKBP AND RAPAMYCIN (C,G) AND THE N-TERMINAL HEAT REPEATS OF MTOR (1-4), HAVE BEEN NUMBERED FROM 100 AT EACH BREAK TO INDICATE LACK OF SEQUENCE - DENSITY CERTAINTY. CHAINS 1-4 CORRESPOND TO THE N-TERMINAL HEAT REPEAT DOMAINS OF MTOR. WE PROPOSE A TOPOLOGY IN THE CORRESPONDING PAPER (1-2-B AND 3-4-F), BUT GIVEN THE FACT THAT THEIR TOPOLOGY CANNOT BE ASSIGNED DEFINITIVELY THEY ARE REPRESENTED AS A SEPARATE CHAIN FOR EACH DOMAIN. THE FITTING OF CRYSTAL STRUCTURES FOR THE MTOR FAT AND PIKK DOMAINS, MLST8, RAPTOR AND FKBP ALLOWS THEIR DENSITY TO BE ASSIGNED DEFINITIVELY, AND THEIR CHAINS ARE THEREFORE LETTERED. SUBMISSION BASED ON EXPERIMENTAL DATA FROM EMDB EMD-3213. (DEPOSITION ID: 13912). 対称性のタイプ: POINT | ||||||||||||||||
| 原子モデル構築 | プロトコル: RIGID BODY FIT / Target criteria: FSC / 詳細: METHOD--RIGID BODY | ||||||||||||||||
| 精密化 | 最高解像度: 5.9 Å | ||||||||||||||||
| 精密化ステップ | サイクル: LAST / 最高解像度: 5.9 Å
|
 ムービー
ムービー コントローラー
コントローラー












 PDBj
PDBj











