[English] 日本語
 Yorodumi
Yorodumi- PDB-5d9i: SV40 Large T antigen origin binding domain bound to artificial DN... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5d9i | ||||||
|---|---|---|---|---|---|---|---|
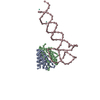
| Title | SV40 Large T antigen origin binding domain bound to artificial DNA fork | ||||||
 Components Components |
| ||||||
 Keywords Keywords | replication/dna / origin binding domain / replication / sv40 / replication-dna complex | ||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host JAK-STAT cascade via inhibition of JAK1 activity / bidirectional double-stranded viral DNA replication / viral DNA genome replication / DNA 3'-5' helicase / symbiont-mediated perturbation of host cell cycle G1/S transition checkpoint / DNA replication origin binding / helicase activity / single-stranded DNA binding / double-stranded DNA binding / symbiont-mediated perturbation of host ubiquitin-like protein modification ...symbiont-mediated suppression of host JAK-STAT cascade via inhibition of JAK1 activity / bidirectional double-stranded viral DNA replication / viral DNA genome replication / DNA 3'-5' helicase / symbiont-mediated perturbation of host cell cycle G1/S transition checkpoint / DNA replication origin binding / helicase activity / single-stranded DNA binding / double-stranded DNA binding / symbiont-mediated perturbation of host ubiquitin-like protein modification / DNA replication / symbiont-mediated suppression of host innate immune response / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / host cell nucleus / ATP hydrolysis activity / zinc ion binding / ATP binding / identical protein binding Similarity search - Function | ||||||
| Biological species |  Simian virus 40 Simian virus 40synthetic construct (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.7 Å MOLECULAR REPLACEMENT / Resolution: 1.7 Å | ||||||
 Authors Authors | Meinke, G. / Bohm, A. / Bullock, P.A. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: J. Virol. / Year: 2011 Journal: J. Virol. / Year: 2011Title: Structure-based analysis of the interaction between the simian virus 40 T-antigen origin binding domain and single-stranded DNA. Authors: Meinke, G. / Phelan, P.J. / Fradet-Turcotte, A. / Bohm, A. / Archambault, J. / Bullock, P.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5d9i.cif.gz 5d9i.cif.gz | 178.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5d9i.ent.gz pdb5d9i.ent.gz | 137.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5d9i.json.gz 5d9i.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5d9i_validation.pdf.gz 5d9i_validation.pdf.gz | 466.8 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5d9i_full_validation.pdf.gz 5d9i_full_validation.pdf.gz | 468 KB | Display | |
| Data in XML |  5d9i_validation.xml.gz 5d9i_validation.xml.gz | 15.9 KB | Display | |
| Data in CIF |  5d9i_validation.cif.gz 5d9i_validation.cif.gz | 23.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d9/5d9i https://data.pdbj.org/pub/pdb/validation_reports/d9/5d9i ftp://data.pdbj.org/pub/pdb/validation_reports/d9/5d9i ftp://data.pdbj.org/pub/pdb/validation_reports/d9/5d9i | HTTPS FTP |
-Related structure data
| Related structure data |  3qk2C  2fufS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||||||||
| Unit cell |
| ||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: _ / Ens-ID: 1 / Beg auth comp-ID: GLY / Beg label comp-ID: GLY / End auth comp-ID: PRO / End label comp-ID: PRO / Refine code: _ / Auth seq-ID: 129 - 259 / Label seq-ID: 1 - 131
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 15490.752 Da / Num. of mol.: 2 / Fragment: unp residues 131-260 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Simian virus 40 / Plasmid: pGEX-1lambdaT / Details (production host): GST fusion / Production host: Simian virus 40 / Plasmid: pGEX-1lambdaT / Details (production host): GST fusion / Production host:  References: UniProt: P03070, Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement |
|---|
-DNA chain , 2 types, 2 molecules XY
| #2: DNA chain | Mass: 6550.217 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) synthetic construct (others) / Production host: synthetic construct (others) |
|---|---|
| #3: DNA chain | Mass: 6963.529 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.) synthetic construct (others) / Production host: synthetic construct (others) |
-Non-polymers , 4 types, 235 molecules 






| #4: Chemical | | #5: Chemical | #6: Chemical | ChemComp-ACT / | #7: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.18 Å3/Da / Density % sol: 43.67 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 8.5 Details: 100 mM Tris, 300 mM NH4Cl, 28% polyethylene glycol [PEG] 4000, 20 mM CaCl2 PH range: 8.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X29A / Wavelength: 1.0809 Å / Beamline: X29A / Wavelength: 1.0809 Å |
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Mar 31, 2009 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.0809 Å / Relative weight: 1 |
| Reflection | Resolution: 1.7→50 Å / Num. obs: 37425 / % possible obs: 88.8 % / Redundancy: 7.3 % / Net I/σ(I): 15.9 |
| Reflection shell | Resolution: 1.7→1.76 Å / Redundancy: 4.6 % / % possible all: 54.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2FUF Resolution: 1.7→50 Å / Cor.coef. Fo:Fc: 0.965 / Cor.coef. Fo:Fc free: 0.945 / SU B: 5.94 / SU ML: 0.098 / Cross valid method: THROUGHOUT / ESU R: 0.126 / ESU R Free: 0.123 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 35.147 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.7→50 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller









 PDBj
PDBj










































