+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5d3h | ||||||
|---|---|---|---|---|---|---|---|
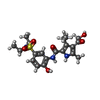
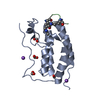
| Title | First bromodomain of BRD4 bound to inhibitor XD29 | ||||||
 Components Components | Bromodomain-containing protein 4 | ||||||
 Keywords Keywords | TRANSCRIPTION / Gene regulation / Bromodomain / Inhibitor | ||||||
| Function / homology |  Function and homology information Function and homology informationhistone H4K8ac reader activity / histone H3K9ac reader activity / RNA polymerase II C-terminal domain binding / histone H3K27ac reader activity / P-TEFb complex binding / negative regulation of DNA damage checkpoint / histone H4 reader activity / histone H4K5ac reader activity / histone H4K12ac reader activity / host-mediated suppression of viral transcription ...histone H4K8ac reader activity / histone H3K9ac reader activity / RNA polymerase II C-terminal domain binding / histone H3K27ac reader activity / P-TEFb complex binding / negative regulation of DNA damage checkpoint / histone H4 reader activity / histone H4K5ac reader activity / histone H4K12ac reader activity / host-mediated suppression of viral transcription / histone H4K16ac reader activity / positive regulation of G2/M transition of mitotic cell cycle / positive regulation of T-helper 17 cell lineage commitment / RNA polymerase II CTD heptapeptide repeat kinase activity / condensed nuclear chromosome / transcription coregulator activity / positive regulation of transcription elongation by RNA polymerase II / p53 binding / chromosome / regulation of inflammatory response / histone binding / Potential therapeutics for SARS / transcription coactivator activity / positive regulation of canonical NF-kappaB signal transduction / transcription cis-regulatory region binding / chromatin remodeling / protein serine/threonine kinase activity / DNA damage response / chromatin binding / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / chromatin / enzyme binding / positive regulation of transcription by RNA polymerase II / nucleoplasm / nucleus Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.7 Å MOLECULAR REPLACEMENT / Resolution: 1.7 Å | ||||||
 Authors Authors | Wohlwend, D. / Huegle, M. / Weitzel, G. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2016 Journal: J.Med.Chem. / Year: 2016Title: 4-Acyl Pyrrole Derivatives Yield Novel Vectors for Designing Inhibitors of the Acetyl-Lysine Recognition Site of BRD4(1). Authors: Hugle, M. / Lucas, X. / Weitzel, G. / Ostrovskyi, D. / Breit, B. / Gerhardt, S. / Einsle, O. / Gunther, S. / Wohlwend, D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5d3h.cif.gz 5d3h.cif.gz | 76.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5d3h.ent.gz pdb5d3h.ent.gz | 57 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5d3h.json.gz 5d3h.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d3/5d3h https://data.pdbj.org/pub/pdb/validation_reports/d3/5d3h ftp://data.pdbj.org/pub/pdb/validation_reports/d3/5d3h ftp://data.pdbj.org/pub/pdb/validation_reports/d3/5d3h | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5d24C  5d25C  5d26C  5d3jC  5d3lC  5d3nC  5d3pC  5d3rC  5d3sC  5d3tC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 15012.301 Da / Num. of mol.: 1 / Fragment: First bromodomain, UNP residues 44-168 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: BRD4, HUNK1 / Production host: Homo sapiens (human) / Gene: BRD4, HUNK1 / Production host:  |
|---|---|
| #2: Chemical | ChemComp-57G / |
| #3: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.86 Å3/Da / Density % sol: 34.03 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / Details: PEG3350, Bis-tris |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU MICROMAX-007 HF / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU MICROMAX-007 HF / Wavelength: 1.5418 Å |
| Detector | Type: RIGAKU SATURN 944+ / Detector: CCD / Date: Jun 24, 2013 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.7→27.1 Å / Num. obs: 11275 / % possible obs: 88.4 % / Redundancy: 2.7 % / Rmerge(I) obs: 0.046 / Net I/σ(I): 10.9 |
| Reflection shell | Resolution: 1.7→1.73 Å / Rmerge(I) obs: 0.266 / Mean I/σ(I) obs: 2.4 / Num. unique all: 475 / % possible all: 74.5 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: in-house model of apo-BRD4(1) Resolution: 1.7→27.1 Å / Cor.coef. Fo:Fc: 0.96 / Cor.coef. Fo:Fc free: 0.938 / SU B: 5.612 / SU ML: 0.082 / Cross valid method: THROUGHOUT / ESU R Free: 0.128 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 14.789 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 1.7→27.1 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



















 PDBj
PDBj