| Entry | Database: PDB / ID: 4wes
|
|---|
| Title | Nitrogenase molybdenum-iron protein from Clostridium pasteurianum at 1.08 A resolution |
|---|
 Components Components | (Nitrogenase molybdenum-iron protein ...) x 2 |
|---|
 Keywords Keywords | OXIDOREDUCTASE / nitrogen fixation |
|---|
| Function / homology |  Function and homology information Function and homology information
molybdenum-iron nitrogenase complex / nitrogenase / nitrogenase activity / nitrogen fixation / iron-sulfur cluster binding / ATP binding / metal ion bindingSimilarity search - Function Nitrogenase Molybdenum-iron Protein, subunit B, domain 4 / Nitrogenase Molybdenum-iron Protein, subunit B; domain 4 / Nitrogenase molybdenum-iron protein alpha chain / Nitrogenase molybdenum-iron protein beta chain / Nitrogenase component 1, alpha chain / Nitrogenase component 1, conserved site / Nitrogenases component 1 alpha and beta subunits signature 2. / Nitrogenases component 1 alpha and beta subunits signature 1. / : / Nitrogenase/oxidoreductase, component 1 ...Nitrogenase Molybdenum-iron Protein, subunit B, domain 4 / Nitrogenase Molybdenum-iron Protein, subunit B; domain 4 / Nitrogenase molybdenum-iron protein alpha chain / Nitrogenase molybdenum-iron protein beta chain / Nitrogenase component 1, alpha chain / Nitrogenase component 1, conserved site / Nitrogenases component 1 alpha and beta subunits signature 2. / Nitrogenases component 1 alpha and beta subunits signature 1. / : / Nitrogenase/oxidoreductase, component 1 / Nitrogenase component 1 type Oxidoreductase / Nitrogenase molybdenum iron protein domain / Up-down Bundle / Rossmann fold / 3-Layer(aba) Sandwich / Mainly Alpha / Alpha BetaSimilarity search - Domain/homology FE(8)-S(7) CLUSTER / : / 3-HYDROXY-3-CARBOXY-ADIPIC ACID / Chem-ICS / Nitrogenase molybdenum-iron protein alpha chain / Nitrogenase molybdenum-iron protein beta chainSimilarity search - Component |
|---|
| Biological species |  Clostridium pasteurianum (bacteria) Clostridium pasteurianum (bacteria) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MAD / Resolution: 1.08 Å MAD / Resolution: 1.08 Å |
|---|
 Authors Authors | Zhang, L.M. / Morrison, C.N. / Kaiser, J.T. / Rees, D.C. |
|---|
| Funding support |  United States, United States,  Canada, 3items Canada, 3items | Organization | Grant number | Country |
|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | GM45162 |  United States United States | | Natural Sciences and Engineering Research Council (NSERC, Canada) | |  Canada Canada | | National Science Foundation (NSF, United States) | DGE-1144469 |  United States United States |
|
|---|
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 2015 Journal: Acta Crystallogr.,Sect.D / Year: 2015
Title: Nitrogenase MoFe protein from Clostridium pasteurianum at 1.08 angstrom resolution: comparison with the Azotobacter vinelandii MoFe protein.
Authors: Zhang, L.M. / Morrison, C.N. / Kaiser, J.T. / Rees, D.C. |
|---|
| History | | Deposition | Sep 10, 2014 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Feb 11, 2015 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Feb 25, 2015 | Group: Database references |
|---|
| Revision 1.2 | Sep 27, 2017 | Group: Advisory / Author supporting evidence ...Advisory / Author supporting evidence / Derived calculations / Other / Refinement description / Source and taxonomy
Category: entity_src_nat / pdbx_audit_support ...entity_src_nat / pdbx_audit_support / pdbx_database_status / pdbx_struct_oper_list / pdbx_validate_close_contact / software
Item: _entity_src_nat.pdbx_alt_source_flag / _pdbx_audit_support.funding_organization ..._entity_src_nat.pdbx_alt_source_flag / _pdbx_audit_support.funding_organization / _pdbx_database_status.pdb_format_compatible / _pdbx_struct_oper_list.symmetry_operation |
|---|
| Revision 1.3 | Nov 27, 2019 | Group: Author supporting evidence / Category: pdbx_audit_support / Item: _pdbx_audit_support.funding_organization |
|---|
| Revision 1.4 | Dec 27, 2023 | Group: Data collection / Database references ...Data collection / Database references / Derived calculations / Refinement description
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / diffrn_radiation_wavelength / pdbx_struct_conn_angle / refine_hist / struct_conn
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_struct_conn_angle.ptnr1_auth_asym_id / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr2_auth_asym_id / _pdbx_struct_conn_angle.ptnr2_auth_comp_id / _pdbx_struct_conn_angle.ptnr2_auth_seq_id / _pdbx_struct_conn_angle.ptnr2_label_alt_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_struct_conn_angle.ptnr2_label_atom_id / _pdbx_struct_conn_angle.ptnr2_label_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_asym_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_alt_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _refine_hist.number_atoms_solvent / _refine_hist.number_atoms_total / _refine_hist.pdbx_number_atoms_ligand / _refine_hist.pdbx_number_atoms_nucleic_acid / _refine_hist.pdbx_number_atoms_protein / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_ptnr1_label_alt_id / _struct_conn.pdbx_ptnr2_label_alt_id / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Clostridium pasteurianum (bacteria)
Clostridium pasteurianum (bacteria) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MAD / Resolution: 1.08 Å
MAD / Resolution: 1.08 Å  Authors
Authors United States,
United States,  Canada, 3items
Canada, 3items  Citation
Citation Journal: Acta Crystallogr.,Sect.D / Year: 2015
Journal: Acta Crystallogr.,Sect.D / Year: 2015 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4wes.cif.gz
4wes.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4wes.ent.gz
pdb4wes.ent.gz PDB format
PDB format 4wes.json.gz
4wes.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/we/4wes
https://data.pdbj.org/pub/pdb/validation_reports/we/4wes ftp://data.pdbj.org/pub/pdb/validation_reports/we/4wes
ftp://data.pdbj.org/pub/pdb/validation_reports/we/4wes Links
Links Assembly
Assembly
 Components
Components Clostridium pasteurianum (bacteria) / Strain: DSM 525 / References: UniProt: P00467, nitrogenase
Clostridium pasteurianum (bacteria) / Strain: DSM 525 / References: UniProt: P00467, nitrogenase Clostridium pasteurianum (bacteria) / Strain: DSM 525 / References: UniProt: P11347, nitrogenase
Clostridium pasteurianum (bacteria) / Strain: DSM 525 / References: UniProt: P11347, nitrogenase
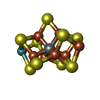

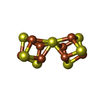


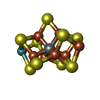



 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SSRL
SSRL  / Beamline: BL12-2 / Wavelength: 0.8856, 1.734, 1.742
/ Beamline: BL12-2 / Wavelength: 0.8856, 1.734, 1.742 Processing
Processing MAD / Resolution: 1.08→38.93 Å / Cor.coef. Fo:Fc: 0.988 / Cor.coef. Fo:Fc free: 0.983 / SU B: 0.854 / SU ML: 0.017 / Cross valid method: THROUGHOUT / ESU R: 0.02 / ESU R Free: 0.022 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
MAD / Resolution: 1.08→38.93 Å / Cor.coef. Fo:Fc: 0.988 / Cor.coef. Fo:Fc free: 0.983 / SU B: 0.854 / SU ML: 0.017 / Cross valid method: THROUGHOUT / ESU R: 0.02 / ESU R Free: 0.022 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS Movie
Movie Controller
Controller












 PDBj
PDBj









