| Entry | Database: PDB / ID: 4rut
|
|---|
| Title | crystal structure of murine cyclooxygenase-2 with 13-methyl-arachidonic Acid |
|---|
 Components Components | Prostaglandin G/H synthase 2 |
|---|
 Keywords Keywords | OXIDOREDUCTASE / PROTEIN-LIGAND COMPLEX / PROSTAGLANDIN-ENDOPEROXIDE SYNTHASE / FATTY ACID ANALOG / GLYCOSYLATION / MEMBRANE |
|---|
| Function / homology |  Function and homology information Function and homology information
Biosynthesis of DHA-derived SPMs / Biosynthesis of EPA-derived SPMs / Biosynthesis of DPAn-3 SPMs / Biosynthesis of electrophilic ω-3 PUFA oxo-derivatives / Synthesis of 15-eicosatetraenoic acid derivatives / cellular response to non-ionic osmotic stress / : / Synthesis of Prostaglandins (PG) and Thromboxanes (TX) / prostaglandin-endoperoxide synthase / prostaglandin-endoperoxide synthase activity ...Biosynthesis of DHA-derived SPMs / Biosynthesis of EPA-derived SPMs / Biosynthesis of DPAn-3 SPMs / Biosynthesis of electrophilic ω-3 PUFA oxo-derivatives / Synthesis of 15-eicosatetraenoic acid derivatives / cellular response to non-ionic osmotic stress / : / Synthesis of Prostaglandins (PG) and Thromboxanes (TX) / prostaglandin-endoperoxide synthase / prostaglandin-endoperoxide synthase activity / cyclooxygenase pathway / negative regulation of intrinsic apoptotic signaling pathway in response to osmotic stress / positive regulation of prostaglandin biosynthetic process / regulation of neuroinflammatory response / positive regulation of fever generation / response to selenium ion / prostaglandin secretion / cellular response to fluid shear stress / response to nematode / prostaglandin biosynthetic process / nuclear inner membrane / dioxygenase activity / bone mineralization / decidualization / nuclear outer membrane / keratinocyte differentiation / positive regulation of brown fat cell differentiation / brown fat cell differentiation / embryo implantation / peroxidase activity / regulation of blood pressure / regulation of cell population proliferation / response to oxidative stress / cellular response to hypoxia / neuron projection / heme binding / endoplasmic reticulum membrane / negative regulation of apoptotic process / enzyme binding / protein homodimerization activity / metal ion binding / cytoplasm / cytosolSimilarity search - Function : / Myeloperoxidase, subunit C / Haem peroxidase domain superfamily, animal type / Haem peroxidase, animal-type / Haem peroxidase domain superfamily, animal type / Animal haem peroxidase / Animal heme peroxidase superfamily profile. / Laminin / Laminin / EGF-like domain ...: / Myeloperoxidase, subunit C / Haem peroxidase domain superfamily, animal type / Haem peroxidase, animal-type / Haem peroxidase domain superfamily, animal type / Animal haem peroxidase / Animal heme peroxidase superfamily profile. / Laminin / Laminin / EGF-like domain / Haem peroxidase superfamily / EGF-like domain profile. / EGF-like domain / Ribbon / Orthogonal Bundle / Mainly Beta / Mainly AlphaSimilarity search - Domain/homology |
|---|
| Biological species |   Mus musculus (house mouse) Mus musculus (house mouse) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.16 Å MOLECULAR REPLACEMENT / Resolution: 2.16 Å |
|---|
 Authors Authors | Xu, S. / Kudalkar, S.N. / Banerjee, S. / Makriyannis, A. / Nikas, S.P. / Marnett, L.J. |
|---|
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2015 Journal: J.Biol.Chem. / Year: 2015
Title: 13-methylarachidonic Acid is a positive allosteric modulator of endocannabinoid oxygenation by cyclooxygenase.
Authors: Kudalkar, S.N. / Nikas, S.P. / Kingsley, P.J. / Xu, S. / Galligan, J.J. / Rouzer, C.A. / Banerjee, S. / Ji, L. / Eno, M.R. / Makriyannis, A. / Marnett, L.J. |
|---|
| History | | Deposition | Nov 21, 2014 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Revision 1.0 | Feb 11, 2015 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Apr 15, 2015 | Group: Database references |
|---|
| Revision 1.2 | Nov 20, 2019 | Group: Derived calculations / Category: pdbx_struct_conn_angle / struct_conn |
|---|
| Revision 2.0 | Jul 29, 2020 | Group: Atomic model / Data collection ...Atomic model / Data collection / Derived calculations / Structure summary
Category: atom_site / chem_comp ...atom_site / chem_comp / entity / pdbx_branch_scheme / pdbx_chem_comp_identifier / pdbx_entity_branch / pdbx_entity_branch_descriptor / pdbx_entity_branch_link / pdbx_entity_branch_list / pdbx_entity_nonpoly / pdbx_nonpoly_scheme / pdbx_struct_assembly_gen / pdbx_struct_conn_angle / struct_asym / struct_conn / struct_site / struct_site_gen
Item: _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ..._atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_asym_id / _atom_site.auth_atom_id / _atom_site.auth_comp_id / _atom_site.auth_seq_id / _atom_site.label_asym_id / _atom_site.label_atom_id / _atom_site.label_comp_id / _atom_site.label_entity_id / _atom_site.pdbx_formal_charge / _atom_site.type_symbol / _chem_comp.mon_nstd_flag / _chem_comp.name / _chem_comp.type / _pdbx_entity_nonpoly.entity_id / _pdbx_entity_nonpoly.name / _pdbx_struct_assembly_gen.asym_id_list / _pdbx_struct_conn_angle.ptnr1_label_asym_id / _pdbx_struct_conn_angle.ptnr2_label_asym_id / _pdbx_struct_conn_angle.ptnr3_label_asym_id / _struct_conn.conn_type_id / _struct_conn.id / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.pdbx_role / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id
Description: Carbohydrate remediation / Provider: repository / Type: Remediation |
|---|
| Revision 2.1 | Sep 20, 2023 | Group: Data collection / Database references ...Data collection / Database references / Refinement description / Structure summary
Category: chem_comp / chem_comp_atom ...chem_comp / chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model
Item: _chem_comp.pdbx_synonyms / _database_2.pdbx_DOI / _database_2.pdbx_database_accession |
|---|
| Revision 2.2 | Nov 20, 2024 | Group: Data collection / Structure summary
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / pdbx_entry_details / pdbx_modification_feature / pdbx_validate_chiral
Item: _chem_comp_atom.pdbx_aromatic_flag / _chem_comp_atom.pdbx_stereo_config / _chem_comp_bond.pdbx_aromatic_flag |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.16 Å
MOLECULAR REPLACEMENT / Resolution: 2.16 Å  Authors
Authors Citation
Citation Journal: J.Biol.Chem. / Year: 2015
Journal: J.Biol.Chem. / Year: 2015 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4rut.cif.gz
4rut.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4rut.ent.gz
pdb4rut.ent.gz PDB format
PDB format 4rut.json.gz
4rut.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ru/4rut
https://data.pdbj.org/pub/pdb/validation_reports/ru/4rut ftp://data.pdbj.org/pub/pdb/validation_reports/ru/4rut
ftp://data.pdbj.org/pub/pdb/validation_reports/ru/4rut
 Links
Links Assembly
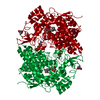
Assembly


 Components
Components








 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 24-ID-C / Wavelength: 0.97918 Å
/ Beamline: 24-ID-C / Wavelength: 0.97918 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller





















 PDBj
PDBj





