+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4m4c | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Rhodostomin ARGDP mutant | ||||||
 Components Components | Zinc metalloproteinase/disintegrin | ||||||
 Keywords Keywords | TOXIN / Rhodostomin / disintegrin / integrin / ARGDP / Loss-of-function | ||||||
| Function / homology |  Function and homology information Function and homology informationHydrolases; Acting on peptide bonds (peptidases); Metalloendopeptidases / metalloendopeptidase activity / toxin activity / proteolysis / extracellular region / metal ion binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  Calloselasma rhodostoma (Malayan pit viper) Calloselasma rhodostoma (Malayan pit viper) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | ||||||
 Authors Authors | Chang, Y.T. / Jeng, W.Y. / Shiu, J.H. / Chen, C.Y. / Chuang, W.J. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: Effect of C-terminal proline residue adjacent to the RGD motif in rhodostomin on its activity and structure Authors: Chang, Y.T. / Jeng, W.Y. / Shiu, J.H. / Chen, C.Y. / Chuang, W.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4m4c.cif.gz 4m4c.cif.gz | 113.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4m4c.ent.gz pdb4m4c.ent.gz | 90 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4m4c.json.gz 4m4c.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/m4/4m4c https://data.pdbj.org/pub/pdb/validation_reports/m4/4m4c ftp://data.pdbj.org/pub/pdb/validation_reports/m4/4m4c ftp://data.pdbj.org/pub/pdb/validation_reports/m4/4m4c | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3uciS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 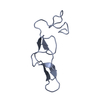
| ||||||||||||||||||||||||||||||
| 2 | 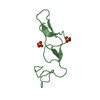
| ||||||||||||||||||||||||||||||
| 3 | 
| ||||||||||||||||||||||||||||||
| 4 | 
| ||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: 1 / Ens-ID: 1 / Beg auth comp-ID: CYS / Beg label comp-ID: CYS / End auth comp-ID: ARG / End label comp-ID: ARG / Refine code: 4 / Auth seq-ID: 4 - 40 / Label seq-ID: 4 - 40
|
- Components
Components
| #1: Protein | Mass: 7281.243 Da / Num. of mol.: 4 / Fragment: UNP residues 408-475 / Mutation: P48A, M52P Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Calloselasma rhodostoma (Malayan pit viper) Calloselasma rhodostoma (Malayan pit viper)Gene: RHOD / Plasmid: pPICZalphaA / Production host:  Pichia pastoris (fungus) / Strain (production host): X-33 Pichia pastoris (fungus) / Strain (production host): X-33References: UniProt: P30403, Hydrolases; Acting on peptide bonds (peptidases); Metalloendopeptidases #2: Chemical | ChemComp-SO4 / #3: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.96 Å3/Da / Density % sol: 37.15 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / pH: 8 Details: 20% PEG4000, 0.2M Ammonium sulfate, 5% PEG3350, 2% PEG200, 0.5% 2-propanol(External) , pH 8.0, VAPOR DIFFUSION, SITTING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSRRC NSRRC  / Beamline: BL13C1 / Wavelength: 0.97622 Å / Beamline: BL13C1 / Wavelength: 0.97622 Å |
| Detector | Type: ADSC QUANTUM 210 / Detector: CCD / Date: Jul 2, 2013 Details: Vertically Focusing Mirror, Horizontally Focusing Single Crystal Si(111) Bent Monochromator |
| Radiation | Monochromator: Horizontally Focusing Single Crystal Monochromator Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97622 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→30 Å / Num. all: 20296 / Num. obs: 19633 / % possible obs: 96.7 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 1 / Redundancy: 3.7 % / Rmerge(I) obs: 0.059 / Net I/σ(I): 23.9 |
| Reflection shell | Resolution: 1.8→1.86 Å / Redundancy: 3.3 % / Rmerge(I) obs: 0.459 / Mean I/σ(I) obs: 2.3 / % possible all: 91.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3UCI Resolution: 1.8→23.58 Å / Cor.coef. Fo:Fc: 0.962 / Cor.coef. Fo:Fc free: 0.938 / SU B: 9.14 / SU ML: 0.126 / Isotropic thermal model: Isotropic with TLS / Cross valid method: THROUGHOUT / ESU R: 0.843 / ESU R Free: 0.146 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 33.953 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→23.58 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller







 PDBj
PDBj





