Entry Database : PDB / ID : 4ipzTitle SmBz bound to Cyclophilin A Peptidyl-prolyl cis-trans isomerase A cyclosporine SmBz-CsA Keywords / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Tolypocladium inflatum (fungus)Method / / / Resolution : 1.67 Å Authors Price, A.J. / Jacques, D.A. / James, L.C. Journal : Nature / Year : 2013Title : HIV-1 evades innate immune recognition through specific cofactor recruitment.Authors : Rasaiyaah, J. / Tan, C.P. / Fletcher, A.J. / Price, A.J. / Blondeau, C. / Hilditch, L. / Jacques, D.A. / Selwood, D.L. / James, L.C. / Noursadeghi, M. / Towers, G.J. History Deposition Jan 10, 2013 Deposition site / Processing site Revision 1.0 Nov 6, 2013 Provider / Type Revision 1.1 Dec 4, 2013 Group Revision 1.2 Aug 29, 2018 Group / Source and taxonomy / Structure summaryCategory / entity_src_nat / pdbx_entity_src_synItem / _entity.pdbx_mutation / _entity.src_methodRevision 1.3 Sep 20, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model / struct_conn / struct_ref_seq_dif / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_ref_seq_dif.details / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id Revision 2.0 Nov 15, 2023 Group / Data collection / Derived calculationsCategory atom_site / atom_site_anisotrop ... atom_site / atom_site_anisotrop / chem_comp_atom / chem_comp_bond / struct_conn Item _atom_site.auth_atom_id / _atom_site.label_atom_id ... _atom_site.auth_atom_id / _atom_site.label_atom_id / _atom_site_anisotrop.pdbx_auth_atom_id / _atom_site_anisotrop.pdbx_label_atom_id / _chem_comp_atom.atom_id / _chem_comp_bond.atom_id_1 / _chem_comp_bond.atom_id_2 / _struct_conn.pdbx_leaving_atom_flag Revision 3.0 May 8, 2024 Group / Category / atom_site_anisotropItem _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ... _atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_atom_id / _atom_site.label_atom_id / _atom_site.type_symbol / _atom_site_anisotrop.U[1][1] / _atom_site_anisotrop.U[1][2] / _atom_site_anisotrop.U[1][3] / _atom_site_anisotrop.U[2][2] / _atom_site_anisotrop.U[2][3] / _atom_site_anisotrop.U[3][3] / _atom_site_anisotrop.pdbx_auth_atom_id / _atom_site_anisotrop.pdbx_label_atom_id / _atom_site_anisotrop.type_symbol
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) Tolypocladium inflatum (fungus)
Tolypocladium inflatum (fungus) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.67 Å
molecular replacement / Resolution: 1.67 Å  Authors
Authors Citation
Citation Journal: Nature / Year: 2013
Journal: Nature / Year: 2013 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4ipz.cif.gz
4ipz.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4ipz.ent.gz
pdb4ipz.ent.gz PDB format
PDB format 4ipz.json.gz
4ipz.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ip/4ipz
https://data.pdbj.org/pub/pdb/validation_reports/ip/4ipz ftp://data.pdbj.org/pub/pdb/validation_reports/ip/4ipz
ftp://data.pdbj.org/pub/pdb/validation_reports/ip/4ipz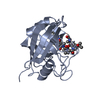
 Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: PPIA, CYPA / Production host:
Homo sapiens (human) / Gene: PPIA, CYPA / Production host: 

 Type: Cyclic peptide / Class: Immunosuppressant / Mass: 1354.757 Da / Num. of mol.: 1 / Mutation: (sar)7(1JM) / Source method: obtained synthetically / Details: CsA has been modified at position 4 (sarcosine) / Source: (synth.)
Type: Cyclic peptide / Class: Immunosuppressant / Mass: 1354.757 Da / Num. of mol.: 1 / Mutation: (sar)7(1JM) / Source method: obtained synthetically / Details: CsA has been modified at position 4 (sarcosine) / Source: (synth.)  Tolypocladium inflatum (fungus) / References: NOR: NOR00033, SmBz, cyclosporin A derivative
Tolypocladium inflatum (fungus) / References: NOR: NOR00033, SmBz, cyclosporin A derivative X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation ROTATING ANODE / Type: RIGAKU FR-E SUPERBRIGHT / Wavelength: 1.5418 Å
ROTATING ANODE / Type: RIGAKU FR-E SUPERBRIGHT / Wavelength: 1.5418 Å molecular replacement
molecular replacement Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller









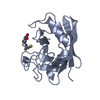



 PDBj
PDBj














