[English] 日本語
 Yorodumi
Yorodumi- PDB-4imq: Structural Basis of Substrate Specificity and Protease Inhibition... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4imq | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structural Basis of Substrate Specificity and Protease Inhibition in Norwalk Virus | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | hydrolase/hydrolase inhibitor / Protease / hydrolase-hydrolase inhibitor complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationcalicivirin / host cell Golgi membrane / ribonucleoside triphosphate phosphatase activity / nucleoside-triphosphate phosphatase / RNA helicase activity / host cell perinuclear region of cytoplasm / host cell endoplasmic reticulum membrane / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication ...calicivirin / host cell Golgi membrane / ribonucleoside triphosphate phosphatase activity / nucleoside-triphosphate phosphatase / RNA helicase activity / host cell perinuclear region of cytoplasm / host cell endoplasmic reticulum membrane / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / proteolysis / RNA binding / extracellular region / ATP binding / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |  Norovirus Hu/1968/US Norovirus Hu/1968/USsynthetic construct (others) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.5 Å MOLECULAR REPLACEMENT / Resolution: 1.5 Å | |||||||||
 Authors Authors | Prasad, B.V.V. / Muhaxhiri, Z. / Deng, L. / Shanker, S. / Sankaran, B. / Estes, M.K. / Palzkill, T. / Song, Y. | |||||||||
 Citation Citation |  Journal: J.Virol. / Year: 2013 Journal: J.Virol. / Year: 2013Title: Structural basis of substrate specificity and protease inhibition in norwalk virus. Authors: Muhaxhiri, Z. / Deng, L. / Shanker, S. / Sankaran, B. / Estes, M.K. / Palzkill, T. / Song, Y. / Prasad, B.V. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4imq.cif.gz 4imq.cif.gz | 88.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4imq.ent.gz pdb4imq.ent.gz | 66.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4imq.json.gz 4imq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/im/4imq https://data.pdbj.org/pub/pdb/validation_reports/im/4imq ftp://data.pdbj.org/pub/pdb/validation_reports/im/4imq ftp://data.pdbj.org/pub/pdb/validation_reports/im/4imq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4imzC  4in1C  4in2C  4inhC  2fyqS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 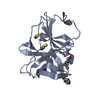
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||
| Unit cell |
| ||||||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 19310.180 Da / Num. of mol.: 1 / Fragment: norwalk virus protease (unp residues 1101-1281) Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Norovirus Hu/1968/US / Strain: GI/Human/United States/Norwalk/1968 / Gene: ORF1 / Production host: Norovirus Hu/1968/US / Strain: GI/Human/United States/Norwalk/1968 / Gene: ORF1 / Production host:  | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #2: Protein/peptide | | ||||||||
| #3: Chemical | | #4: Chemical | ChemComp-NA / | #5: Water | ChemComp-HOH / | Has protein modification | Y | Sequence details | THE UNBOUND FORM OF THE PEPTIDE INHIBITOR SYC8 HAD AN ALDEHYDE GROUP THAT UNDERWENT TO REDUCTION ...THE UNBOUND FORM OF THE PEPTIDE INHIBITOR SYC8 HAD AN ALDEHYDE GROUP THAT UNDERWENT TO REDUCTION UPON REACTION WITH CYS 139 | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.79 Å3/Da / Density % sol: 55.98 % |
|---|---|
| Crystal grow | Temperature: 293 K / pH: 7 Details: 0.1-0.3 M potassium, thiocyanate and 25-30%, polyethylene glycol, monomethyl ether 2,000 , pH 7, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 77 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 19-ID / Wavelength: 0.97921 / Beamline: 19-ID / Wavelength: 0.97921 |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Mar 11, 2011 |
| Radiation | Monochromator: ROSENBAUM-ROCK HIGH-RESOLUTION DOUBLE-CRYSTAL MONOCHROMATOR. LN2 COOLED FIRST CRYSTAL, SAGITTAL FOCUSING 2ND CRYSTAL Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97921 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→50 Å / Num. obs: 36665 / % possible obs: 99.9 % / Observed criterion σ(I): 2 / Redundancy: 15.9 % / Rmerge(I) obs: 0.069 |
| Reflection shell | Resolution: 1.5→1.53 Å / % possible all: 99.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2FYQ Resolution: 1.5→31.59 Å / SU ML: 0.16 / σ(F): 1.34 / Phase error: 16.46 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.95 Å / VDW probe radii: 1.2 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 37.77 Å2 / ksol: 0.34 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→31.59 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj