[English] 日本語
 Yorodumi
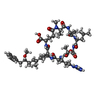
Yorodumi- PDB-4i5l: Structural mechanism of trimeric PP2A holoenzyme involving PR70: ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4i5l | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structural mechanism of trimeric PP2A holoenzyme involving PR70: insight for Cdc6 dephosphorylation | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | HYDROLASE/TOXIN / EF Hand / Phosphatase / CDC6 (Substrate) / HYDROLASE-TOXIN complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of chromosome segregation / cellular response to vasopressin / CDC6 association with the ORC:origin complex / meiotic spindle elongation / Integration of energy metabolism / PP2A-mediated dephosphorylation of key metabolic factors / RNA polymerase II CTD heptapeptide repeat S2 phosphatase activity / RNA polymerase II CTD heptapeptide repeat S7 phosphatase activity / MASTL Facilitates Mitotic Progression / regulation of meiotic cell cycle process involved in oocyte maturation ...positive regulation of chromosome segregation / cellular response to vasopressin / CDC6 association with the ORC:origin complex / meiotic spindle elongation / Integration of energy metabolism / PP2A-mediated dephosphorylation of key metabolic factors / RNA polymerase II CTD heptapeptide repeat S2 phosphatase activity / RNA polymerase II CTD heptapeptide repeat S7 phosphatase activity / MASTL Facilitates Mitotic Progression / regulation of meiotic cell cycle process involved in oocyte maturation / protein phosphatase type 2A complex / mitotic sister chromatid separation / peptidyl-threonine dephosphorylation / protein serine/threonine phosphatase complex / meiotic sister chromatid cohesion, centromeric / INTAC complex / RNA polymerase II CTD heptapeptide repeat S5 phosphatase activity / FAR/SIN/STRIPAK complex / Regulation of glycolysis by fructose 2,6-bisphosphate metabolism / Inhibition of replication initiation of damaged DNA by RB1/E2F1 / female meiotic nuclear division / traversing start control point of mitotic cell cycle / protein phosphatase regulator activity / DNA replication checkpoint signaling / GABA receptor binding / protein antigen binding / APC truncation mutants have impaired AXIN binding / AXIN missense mutants destabilize the destruction complex / Truncations of AMER1 destabilize the destruction complex / ERKs are inactivated / positive regulation of extrinsic apoptotic signaling pathway in absence of ligand / regulation of cyclin-dependent protein serine/threonine kinase activity / mitotic DNA replication checkpoint signaling / Initiation of Nuclear Envelope (NE) Reformation / Beta-catenin phosphorylation cascade / Signaling by GSK3beta mutants / CTNNB1 S33 mutants aren't phosphorylated / CTNNB1 S37 mutants aren't phosphorylated / CTNNB1 S45 mutants aren't phosphorylated / CTNNB1 T41 mutants aren't phosphorylated / Transcription of E2F targets under negative control by DREAM complex / RNA polymerase II transcription initiation surveillance / Co-stimulation by CD28 / regulation of growth / Disassembly of the destruction complex and recruitment of AXIN to the membrane / regulation of mitotic metaphase/anaphase transition / negative regulation of epithelial to mesenchymal transition / Co-inhibition by CTLA4 / Platelet sensitization by LDL / protein dephosphorylation / protein-serine/threonine phosphatase / positive regulation of cytokinesis / spindle midzone / G1/S-Specific Transcription / ERK/MAPK targets / negative regulation of glycolytic process through fructose-6-phosphate / negative regulation of DNA replication / positive regulation of NLRP3 inflammasome complex assembly / mesoderm development / protein serine/threonine phosphatase activity / vascular endothelial cell response to oscillatory fluid shear stress / T cell homeostasis / regulation of cell differentiation / regulation of microtubule polymerization / regulation of G1/S transition of mitotic cell cycle / cellular response to angiotensin / DNA replication origin binding / lateral plasma membrane / DNA replication initiation / Activation of the pre-replicative complex / chromosome, centromeric region / DARPP-32 events / negative regulation of hippo signaling / Activation of ATR in response to replication stress / Cyclin A/B1/B2 associated events during G2/M transition / intercellular bridge / Nonsense Mediated Decay (NMD) enhanced by the Exon Junction Complex (EJC) / spindle assembly / phosphoprotein phosphatase activity / Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of mitotic centrosome proteins and complexes / Recruitment of NuMA to mitotic centrosomes / Mitotic Prometaphase / Anchoring of the basal body to the plasma membrane / protein tyrosine phosphatase activity / negative regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / EML4 and NUDC in mitotic spindle formation / protein serine/threonine kinase binding / Turbulent (oscillatory, disturbed) flow shear stress activates signaling by PIEZO1 and integrins in endothelial cells / AURKA Activation by TPX2 / Resolution of Sister Chromatid Cohesion / meiotic cell cycle / chromosome segregation / Assembly of the pre-replicative complex / RAF activation / Spry regulation of FGF signaling / negative regulation of canonical Wnt signaling pathway / RHO GTPases Activate Formins Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) Microcystis aeruginosa (bacteria) Microcystis aeruginosa (bacteria) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.43 Å MOLECULAR REPLACEMENT / Resolution: 2.43 Å | |||||||||
 Authors Authors | Wlodarchak, N. / Satyshur, K.A. / Guo, F. / Xing, Y. | |||||||||
 Citation Citation |  Journal: Cell Res. / Year: 2013 Journal: Cell Res. / Year: 2013Title: Structure of the Ca(2+)-dependent PP2A heterotrimer and insights into Cdc6 dephosphorylation. Authors: Wlodarchak, N. / Guo, F. / Satyshur, K.A. / Jiang, L. / Jeffrey, P.D. / Sun, T. / Stanevich, V. / Mumby, M.C. / Xing, Y. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4i5l.cif.gz 4i5l.cif.gz | 997.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4i5l.ent.gz pdb4i5l.ent.gz | 825.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4i5l.json.gz 4i5l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/i5/4i5l https://data.pdbj.org/pub/pdb/validation_reports/i5/4i5l ftp://data.pdbj.org/pub/pdb/validation_reports/i5/4i5l ftp://data.pdbj.org/pub/pdb/validation_reports/i5/4i5l | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Serine/threonine-protein phosphatase 2A ... , 3 types, 6 molecules ADBECF
| #1: Protein | Mass: 64906.918 Da / Num. of mol.: 2 / Fragment: PP2A A alpha subunit (9-589) Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PPP2R1A / Plasmid: pCool / Cell line (production host): DH5a / Production host: Homo sapiens (human) / Gene: PPP2R1A / Plasmid: pCool / Cell line (production host): DH5a / Production host:  #2: Protein | Mass: 47227.238 Da / Num. of mol.: 2 Fragment: UNP Q9Y5P8 residues 122-490 and UNP Q99741 residues 70-90 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PPP2R3B, PPP2R3L / Plasmid: PQlinkG / Cell line (production host): DH5a / Production host: Homo sapiens (human) / Gene: PPP2R3B, PPP2R3L / Plasmid: PQlinkG / Cell line (production host): DH5a / Production host:  #3: Protein | Mass: 35780.281 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PPP2CA / Plasmid: FBQH / Cell line (production host): High5 / Production host: Homo sapiens (human) / Gene: PPP2CA / Plasmid: FBQH / Cell line (production host): High5 / Production host:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper)References: UniProt: P67775, protein-serine/threonine phosphatase |
|---|
-Protein/peptide , 1 types, 2 molecules GH
| #4: Protein/peptide |
|---|
-Non-polymers , 5 types, 553 molecules 







| #5: Chemical | ChemComp-PEG / #6: Chemical | ChemComp-CA / #7: Chemical | ChemComp-MN / #8: Chemical | #9: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|---|
| Sequence details | THE LINKER IS DERIVED FROM JMJD6 |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.74 Å3/Da / Density % sol: 55.06 % |
|---|---|
| Crystal grow | Temperature: 292 K / Method: vapor diffusion, hanging drop / pH: 5 Details: 0.1M Na Malonate, 12% PEG 3350 + 6.2mg/mL protein in buffer Tris, 150mM NaCl , pH 5.0, VAPOR DIFFUSION, HANGING DROP, temperature 292K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 21-ID-D / Wavelength: 0.97919 Å / Beamline: 21-ID-D / Wavelength: 0.97919 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: Dec 12, 2011 |
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97919 Å / Relative weight: 1 |
| Reflection | Resolution: 2.43→49.85 Å / Num. all: 125017 / Num. obs: 123017 / % possible obs: 98.4 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 7 % / Rsym value: 0.093 / Net I/σ(I): 19.15 |
| Reflection shell | Resolution: 2.42→2.46 Å / Redundancy: 6.7 % / Mean I/σ(I) obs: 2.74 / Rsym value: 0.615 / % possible all: 89.7 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.43→49.85 Å / Cor.coef. Fo:Fc: 0.954 / Cor.coef. Fo:Fc free: 0.928 / SU B: 14.686 / SU ML: 0.166 / Cross valid method: THROUGHOUT / ESU R: 0.339 / ESU R Free: 0.236 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT MOLECULAR REPLACEMENT / Resolution: 2.43→49.85 Å / Cor.coef. Fo:Fc: 0.954 / Cor.coef. Fo:Fc free: 0.928 / SU B: 14.686 / SU ML: 0.166 / Cross valid method: THROUGHOUT / ESU R: 0.339 / ESU R Free: 0.236 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 42.92 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.43→49.85 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.428→2.491 Å / Total num. of bins used: 20
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj