[English] 日本語
 Yorodumi
Yorodumi- PDB-4hmh: Crystal structure of tankyrase 2 in complex with 7,3-dihydroxyflavone -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4hmh | ||||||
|---|---|---|---|---|---|---|---|
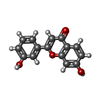
| Title | Crystal structure of tankyrase 2 in complex with 7,3-dihydroxyflavone | ||||||
 Components Components | (Tankyrase-2) x 2 | ||||||
 Keywords Keywords | TRANSFERASE / PROTEIN-LIGAND COMPLEX / DIPHTHERIA TOXIN LIKE FOLD / ADP-RIBOSYLATION | ||||||
| Function / homology |  Function and homology information Function and homology informationXAV939 stabilizes AXIN / positive regulation of telomere capping / NAD+ ADP-ribosyltransferase / protein auto-ADP-ribosylation / protein localization to chromosome, telomeric region / negative regulation of telomere maintenance via telomere lengthening / NAD+-protein-aspartate ADP-ribosyltransferase activity / protein poly-ADP-ribosylation / NAD+-protein-glutamate ADP-ribosyltransferase activity / NAD+-protein mono-ADP-ribosyltransferase activity ...XAV939 stabilizes AXIN / positive regulation of telomere capping / NAD+ ADP-ribosyltransferase / protein auto-ADP-ribosylation / protein localization to chromosome, telomeric region / negative regulation of telomere maintenance via telomere lengthening / NAD+-protein-aspartate ADP-ribosyltransferase activity / protein poly-ADP-ribosylation / NAD+-protein-glutamate ADP-ribosyltransferase activity / NAD+-protein mono-ADP-ribosyltransferase activity / pericentriolar material / Transferases; Glycosyltransferases; Pentosyltransferases / NAD+ poly-ADP-ribosyltransferase activity / positive regulation of telomere maintenance via telomerase / nucleotidyltransferase activity / TCF dependent signaling in response to WNT / Degradation of AXIN / Wnt signaling pathway / Regulation of PTEN stability and activity / protein polyubiquitination / positive regulation of canonical Wnt signaling pathway / nuclear envelope / chromosome, telomeric region / Ub-specific processing proteases / Golgi membrane / perinuclear region of cytoplasm / enzyme binding / metal ion binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | ||||||
 Authors Authors | Narwal, M. / Haikarainen, T. / Lehtio, L. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2013 Journal: J.Med.Chem. / Year: 2013Title: Screening and structural analysis of flavones inhibiting tankyrases. Authors: Narwal, M. / Haikarainen, T. / Fallarero, A. / Vuorela, P.M. / Lehtio, L. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4hmh.cif.gz 4hmh.cif.gz | 60.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4hmh.ent.gz pdb4hmh.ent.gz | 41.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4hmh.json.gz 4hmh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hm/4hmh https://data.pdbj.org/pub/pdb/validation_reports/hm/4hmh ftp://data.pdbj.org/pub/pdb/validation_reports/hm/4hmh ftp://data.pdbj.org/pub/pdb/validation_reports/hm/4hmh | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4hkiC  4hkkC  4hknC  4hl5C  4hlfC  4hlgC  4hlhC  4hlkC  4hlmC  3u9hS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein / Protein/peptide , 2 types, 2 molecules AC
| #1: Protein | Mass: 21824.545 Da / Num. of mol.: 1 / Fragment: C-terminal fragment, UNP residues 946-1113 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TNKS2, PARP5B, TANK2, TNKL / Plasmid: pNIC-Bsa4 / Production host: Homo sapiens (human) / Gene: TNKS2, PARP5B, TANK2, TNKL / Plasmid: pNIC-Bsa4 / Production host:  |
|---|---|
| #2: Protein/peptide | Mass: 5493.216 Da / Num. of mol.: 1 / Fragment: C-terminal fragment, UNP residues 1114-1162 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TNKS2, PARP5B, TANK2, TNKL / Plasmid: pNIC-Bsa4 / Production host: Homo sapiens (human) / Gene: TNKS2, PARP5B, TANK2, TNKL / Plasmid: pNIC-Bsa4 / Production host:  |
-Non-polymers , 6 types, 45 molecules 









| #3: Chemical | ChemComp-ZN / | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| #4: Chemical | | #5: Chemical | ChemComp-GOL / | #6: Chemical | ChemComp-F94 / | #7: Chemical | ChemComp-PEG / | #8: Water | ChemComp-HOH / | |
-Details
| Sequence details | AUTHOR STATES THAT THE BACTERIAL EXPRESSED TANKYRASE 2 CATALYTIC DOMAIN (UNP RESIDUES 946-1162) WAS ...AUTHOR STATES THAT THE BACTERIAL EXPRESSED TANKYRASE 2 CATALYTIC DOMAIN (UNP RESIDUES 946-1162) WAS CLEAVED WITH CHYMOTRYPS |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.42 Å3/Da / Density % sol: 49.27 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: 0.2 M LiSO4, 0.1 M Tris HCl 24 % PEG3350 , pH 8.5, VAPOR DIFFUSION, SITTING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: OTHER / Wavelength: 1.54058 Å ROTATING ANODE / Type: OTHER / Wavelength: 1.54058 Å |
| Detector | Type: Platinum 135 CCD / Detector: CCD / Date: Jun 29, 2012 |
| Radiation | Monochromator: Helios MX / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.54058 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→58.33 Å / Num. obs: 12517 / % possible obs: 99.7 % / Observed criterion σ(F): 0 / Observed criterion σ(I): -3 / Redundancy: 3.47 % / Rmerge(I) obs: 0.1475 / Net I/σ(I): 8.63 |
| Reflection shell | Resolution: 2.3→2.36 Å / Redundancy: 2.75 % / Rmerge(I) obs: 0.35 / Mean I/σ(I) obs: 2.64 / Num. unique all: 2082 / % possible all: 99.6 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3U9H Resolution: 2.3→58.33 Å / Cor.coef. Fo:Fc: 0.919 / Cor.coef. Fo:Fc free: 0.881 / SU B: 7.039 / SU ML: 0.166 / Cross valid method: THROUGHOUT / ESU R: 0.346 / ESU R Free: 0.256 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 15.211 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.3→58.33 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.3→2.36 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller





















 PDBj
PDBj








