[English] 日本語
 Yorodumi
Yorodumi- PDB-4gzw: N2 neuraminidase D151G mutant of A/Tanzania/205/2010 H3N2 in comp... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4gzw | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | N2 neuraminidase D151G mutant of A/Tanzania/205/2010 H3N2 in complex with avian sialic acid receptor | |||||||||
 Components Components | neuraminidase | |||||||||
 Keywords Keywords | VIRAL PROTEIN / beta-propella / influenza virus / neuraminidase / hemagglutinin / hemadsorption / viral infection / sialic acid receptor | |||||||||
| Function / homology |  Function and homology information Function and homology informationexo-alpha-sialidase / exo-alpha-sialidase activity / viral budding from plasma membrane / carbohydrate metabolic process / host cell plasma membrane / virion membrane / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |   Influenza A virus Influenza A virus | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.45 Å MOLECULAR REPLACEMENT / Resolution: 2.45 Å | |||||||||
 Authors Authors | Zhu, X. / Wilson, I.A. | |||||||||
 Citation Citation |  Journal: J.Virol. / Year: 2012 Journal: J.Virol. / Year: 2012Title: Influenza virus neuraminidases with reduced enzymatic activity that avidly bind sialic Acid receptors. Authors: Zhu, X. / McBride, R. / Nycholat, C.M. / Yu, W. / Paulson, J.C. / Wilson, I.A. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4gzw.cif.gz 4gzw.cif.gz | 325.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4gzw.ent.gz pdb4gzw.ent.gz | 265.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4gzw.json.gz 4gzw.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4gzw_validation.pdf.gz 4gzw_validation.pdf.gz | 3.7 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4gzw_full_validation.pdf.gz 4gzw_full_validation.pdf.gz | 3.7 MB | Display | |
| Data in XML |  4gzw_validation.xml.gz 4gzw_validation.xml.gz | 60.2 KB | Display | |
| Data in CIF |  4gzw_validation.cif.gz 4gzw_validation.cif.gz | 83.4 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gz/4gzw https://data.pdbj.org/pub/pdb/validation_reports/gz/4gzw ftp://data.pdbj.org/pub/pdb/validation_reports/gz/4gzw ftp://data.pdbj.org/pub/pdb/validation_reports/gz/4gzw | HTTPS FTP |
-Related structure data
| Related structure data |  4gzoSC  4gzpC  4gzqC  4gzsC  4gztC  4gzxC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein | Mass: 43291.316 Da / Num. of mol.: 4 / Fragment: ectodomain Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Influenza A virus / Strain: A/Tanzania/205/2010(H3N2) / Plasmid: PFASTBACHT-A / Production host: Influenza A virus / Strain: A/Tanzania/205/2010(H3N2) / Plasmid: PFASTBACHT-A / Production host:  Trichoplusia Ni (cabbage looper) / References: UniProt: K7N5N3*PLUS Trichoplusia Ni (cabbage looper) / References: UniProt: K7N5N3*PLUS |
|---|
-Sugars , 9 types, 22 molecules 
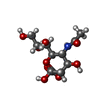

| #2: Polysaccharide | N-acetyl-alpha-neuraminic acid-(2-3)-beta-D-galactopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose / 3'-sialyl-N-acetyllactosamine | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| #3: Polysaccharide | Source method: isolated from a genetically manipulated source #4: Polysaccharide | N-acetyl-alpha-neuraminic acid-(2-3)-beta-D-galactopyranose-(1-3)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #5: Polysaccharide | alpha-D-mannopyranose-(1-3)-beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1- ...alpha-D-mannopyranose-(1-3)-beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #6: Polysaccharide | N-acetyl-alpha-neuraminic acid-(2-3)-beta-D-galactopyranose | Source method: isolated from a genetically manipulated source #7: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #8: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta- ...2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-[alpha-L-fucopyranose-(1-6)]2-acetamido-2-deoxy-beta-D-glucopyranose | Source method: isolated from a genetically manipulated source #9: Sugar | ChemComp-NAG / #11: Sugar | ChemComp-SIA / | |
-Non-polymers , 2 types, 366 molecules 


| #10: Chemical | ChemComp-CA / #12: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|---|
| Sequence details | AUTHORS STATE THAT THE GISAID ACCESSION NUMBER IS EPI279969 FOR THIS SEQUENCE. |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.87 Å3/Da / Density % sol: 57.09 % |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion / pH: 7.5 Details: 0.1 M Hepes, 17-25% PEG 8000, pH 7.5, VAPOR DIFFUSION, temperature 295K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 23-ID-B / Wavelength: 1.0332 Å / Beamline: 23-ID-B / Wavelength: 1.0332 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: Jun 16, 2011 |
| Radiation | Monochromator: Double crystal cryo-cooled Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.0332 Å / Relative weight: 1 |
| Reflection | Resolution: 2.45→50 Å / Num. obs: 67523 / % possible obs: 93.9 % / Observed criterion σ(I): -3 / Redundancy: 3.7 % / Biso Wilson estimate: 37.4 Å2 / Rmerge(I) obs: 0.085 / Net I/σ(I): 20.2 |
| Reflection shell | Resolution: 2.45→2.54 Å / Redundancy: 3.1 % / Rmerge(I) obs: 0.26 / Mean I/σ(I) obs: 4.9 / % possible all: 63.5 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 4GZO Resolution: 2.45→50 Å / Cor.coef. Fo:Fc: 0.941 / Cor.coef. Fo:Fc free: 0.903 / Cross valid method: THROUGHOUT / ESU R: 0.542 / ESU R Free: 0.284 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 28.668 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.45→50 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.45→2.514 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller












 PDBj
PDBj







