[English] 日本語
 Yorodumi
Yorodumi- PDB-4gnm: Structure of rat cytosolic PEPCK Ld_2g in complex with oxalate and GTP -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4gnm | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of rat cytosolic PEPCK Ld_2g in complex with oxalate and GTP | ||||||
 Components Components | Phosphoenolpyruvate carboxykinase, cytosolic [GTP] | ||||||
 Keywords Keywords | LYASE / kinase / gluconeogenesis | ||||||
| Function / homology |  Function and homology information Function and homology informationresponse to methionine / Gluconeogenesis / phosphoenolpyruvate carboxykinase activity / protein serine kinase activity (using GTP as donor) / Transferases; Transferring phosphorus-containing groups; Protein-serine/threonine kinases / cellular response to potassium ion starvation / phosphoenolpyruvate carboxykinase (GTP) / phosphoenolpyruvate carboxykinase (GTP) activity / glycerol biosynthetic process from pyruvate / propionate catabolic process ...response to methionine / Gluconeogenesis / phosphoenolpyruvate carboxykinase activity / protein serine kinase activity (using GTP as donor) / Transferases; Transferring phosphorus-containing groups; Protein-serine/threonine kinases / cellular response to potassium ion starvation / phosphoenolpyruvate carboxykinase (GTP) / phosphoenolpyruvate carboxykinase (GTP) activity / glycerol biosynthetic process from pyruvate / propionate catabolic process / cellular response to raffinose / tricarboxylic acid metabolic process / response to interleukin-6 / regulation of lipid biosynthetic process / cellular response to fructose stimulus / cellular hypotonic salinity response / cellular hypotonic response / carboxylic acid binding / cellular response to phorbol 13-acetate 12-myristate / oxaloacetate metabolic process / hepatocyte differentiation / positive regulation of memory T cell differentiation / cellular hyperosmotic response / glyceraldehyde-3-phosphate biosynthetic process / nucleoside diphosphate kinase activity / response to starvation / cellular hyperosmotic salinity response / response to lipid / cellular response to interleukin-1 / cellular response to dexamethasone stimulus / positive regulation of lipid biosynthetic process / cellular response to retinoic acid / cellular response to glucagon stimulus / cellular response to cAMP / response to activity / gluconeogenesis / response to bacterium / lipid metabolic process / cellular response to glucose stimulus / peptidyl-serine phosphorylation / response to nutrient levels / response to insulin / cellular response to insulin stimulus / glucose metabolic process / cellular response to tumor necrosis factor / GDP binding / glucose homeostasis / manganese ion binding / response to lipopolysaccharide / cellular response to hypoxia / GTP binding / magnesium ion binding / endoplasmic reticulum / positive regulation of transcription by RNA polymerase II / mitochondrion / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 1.5 Å molecular replacement / Resolution: 1.5 Å | ||||||
 Authors Authors | Johnson, T.A. / Holyoak, T. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2012 Journal: Biochemistry / Year: 2012Title: The {Omega}-loop lid domain of phosphoenolpyruvate carboxykinase is essential for catalytic function. Authors: Johnson, T.A. / Holyoak, T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4gnm.cif.gz 4gnm.cif.gz | 154.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4gnm.ent.gz pdb4gnm.ent.gz | 117.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4gnm.json.gz 4gnm.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gn/4gnm https://data.pdbj.org/pub/pdb/validation_reports/gn/4gnm ftp://data.pdbj.org/pub/pdb/validation_reports/gn/4gnm ftp://data.pdbj.org/pub/pdb/validation_reports/gn/4gnm | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4gmmC  4gmuC  4gmwC  4gmzC  4gnlC  4gnoC  4gnpC  4gnqC  3dt2S C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 68546.531 Da / Num. of mol.: 1 / Fragment: SEE REMARK 999 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: P07379, phosphoenolpyruvate carboxykinase (GTP) |
|---|
-Non-polymers , 5 types, 613 molecules 


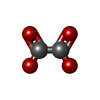





| #2: Chemical | ChemComp-GTP / | ||||||
|---|---|---|---|---|---|---|---|
| #3: Chemical | | #4: Chemical | #5: Chemical | ChemComp-OXL / | #6: Water | ChemComp-HOH / | |
-Details
| Sequence details | RESIDUES 464-474 (ATAAAEHKGK |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.22 Å3/Da / Density % sol: 44.59 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 7.4 Details: 22-26% PEG3350, 0.1 M HEPES, pH 7.4, 10 mM manganese chloride, 10 mM GTP, VAPOR DIFFUSION, HANGING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL7-1 / Wavelength: 0.9 Å / Beamline: BL7-1 / Wavelength: 0.9 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Jan 31, 2011 Details: Flat mirror (vertical focusing), single crystal Si(111) bent monochromator (horizontal focusing) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: Side scattering bent cube-root I-beam single crystal, asymmetric cut 4.965 degrees Protocol: SINGLE WAVELENGTH / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 0.9 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.5→100 Å / Num. obs: 94980 / % possible obs: 96.3 % / Redundancy: 4.4 % / Rmerge(I) obs: 0.112 / Net I/σ(I): 10.6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell |
|
-Phasing
| Phasing | Method:  molecular replacement molecular replacement |
|---|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 3DT2 Resolution: 1.5→26.66 Å / Cor.coef. Fo:Fc: 0.971 / Cor.coef. Fo:Fc free: 0.957 / Occupancy max: 1 / Occupancy min: 0.3 / SU B: 1.891 / SU ML: 0.067 / SU R Cruickshank DPI: 0.0836 / Cross valid method: THROUGHOUT / ESU R: 0.084 / ESU R Free: 0.086 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN USED IF PRESENT IN THE INPUT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 27.082 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→26.66 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.5→1.537 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller












 PDBj
PDBj



