Software Name Version Classification NB data scalingShow phasingShow phasingShow 1.6_289refinementShow 3.11 data extractionShow data collectiondata reduction
Refinement Method to determine structure / Resolution / Occupancy max / Occupancy min / FOM work R set / SU ML / σ(F) / Phase error / Stereochemistry target values Rfactor Num. reflection % reflection Selection details Rfree 0.2302 1029 9.98 % Random Rwork 0.1814 - - - obs 0.1864 10308 96.15 % - all - 10308 - -
Solvent computation Shrinkage radii / VDW probe radii / Solvent model / Bsol 2 / ksol 3 Displacement parameters Biso max 2 / Biso mean 2 / Biso min 2 Baniso -1 Baniso -2 Baniso -3 1- 6.5535 Å2 0 Å2 -0 Å2 2- - 6.5535 Å2 0 Å2 3- - - -13.1069 Å2
Refinement step Cycle / Resolution Protein Nucleic acid Ligand Solvent Total Num. atoms 942 0 6 58 1006
Refine LS restraints Refine-ID Type Dev ideal Number X-RAY DIFFRACTION f_bond_d0.006 947 X-RAY DIFFRACTION f_angle_d0.887 1268 X-RAY DIFFRACTION f_chiral_restr0.06 165 X-RAY DIFFRACTION f_plane_restr0.003 156 X-RAY DIFFRACTION f_dihedral_angle_d15.9 367
LS refinement shell Refine-ID / Total num. of bins used
Resolution (Å)Rfactor Rfree Num. reflection Rfree Rfactor Rwork Num. reflection Rwork Num. reflection all % reflection obs (%)2.2001-2.316 0.2772 135 0.2032 1225 1360 90 2.316-2.461 0.2807 140 0.2083 1249 1389 93 2.461-2.6509 0.2498 145 0.1936 1308 1453 95 2.6509-2.9174 0.207 145 0.182 1328 1473 97 2.9174-3.3388 0.2246 150 0.1779 1357 1507 99 3.3388-4.2039 0.2188 154 0.1557 1375 1529 99 4.2039-26.6084 0.217 160 0.185 1437 1597 99
Refinement TLS params. Method / Refine-ID
Show large table (25 x 20) Hide large table ID L11 (°2 )L12 (°2 )L13 (°2 )L22 (°2 )L23 (°2 )L33 (°2 )S11 (Å °)S12 (Å °)S13 (Å °)S21 (Å °)S22 (Å °)S23 (Å °)S31 (Å °)S32 (Å °)S33 (Å °)T11 (Å2 )T12 (Å2 )T13 (Å2 )T22 (Å2 )T23 (Å2 )T33 (Å2 )Origin x (Å)Origin y (Å)Origin z (Å)1 2.4624 3.0116 -3.2965 3.677 -3.9977 4.409 -0.7234 0.3938 -1.5744 -1.8058 0.2465 -0.8347 2.0193 0.9704 0.0702 0.3192 0.0261 0.093 0.5189 -0.0753 0.3678 32.4342 55.706 42.9845 2 5.2908 -3.9074 1.9083 8.0712 -0.647 3.9463 -0.0488 0.4317 -1.2301 0.5446 0.9003 0.7726 1.0911 0.6378 -0.6334 0.2825 0.0404 -0.0528 0.1915 -0.0595 0.2709 29.3994 55.3289 49.6735 3 4.0907 1.3524 4.994 0.8741 1.5999 6.2563 -0.1981 -0.1281 -1.1392 -0.2369 0.6679 0.1226 -0.8837 0.8587 -0.7109 0.267 0.0097 0.0382 0.5728 0.0125 0.3054 28.6758 53.3447 57.9387 4 5.9661 -4.3881 -2.718 6.086 4.1198 3.5114 -0.1274 -1.2152 -0.1041 0.4991 -0.2192 1.1966 0.569 0.1253 0.253 0.2027 -0.0603 -0.098 0.3503 0.0221 0.2225 26.9233 53.8241 66.0246 5 0.775 -1.6219 -2.1578 6.1421 8.3395 2.0005 0.2838 0.1687 -0.2709 0.0703 -0.7108 0.9171 1.3578 -1.6827 -0.1208 0.1798 -0.051 -0.0508 0.3315 0.1168 0.2853 26.7855 52.7706 74.2199 6 8.1133 1.985 -2.9101 5.3109 -3.62 2.7845 -0.5333 -0.9113 0.6377 0.9965 1.139 0.2218 0.6386 -2.0792 -0.5811 0.3669 0.0582 -0.0425 0.4541 0.0587 0.3764 26.2592 55.4086 85.5489 7 0.5332 -0.7219 -1.9787 6.5821 1.7978 7.4698 0.1821 0.3032 0.1857 0.756 -0.3684 -0.0184 0.1589 -2.2399 0.1376 0.4588 0.2077 -0.0992 0.4742 -0.0987 0.2457 27.9294 58.7443 95.3453 8 0.2187 -0.8847 -0.0556 5.132 -0.0082 0.0482 0.3134 0.1909 0.3912 -0.1439 -0.1934 -0.0355 -0.3706 -0.0138 0.1931 0.3385 0.1862 -0.052 0.2983 -0.0408 0.2659 31.3618 61.9834 105.2104 9 1.7478 -0.0744 0.326 1.9108 0.0909 1.9896 -0.0419 0.0793 0.3292 -0.34 0.1396 -0.108 0.0336 -0.1308 0.1759 0.2083 -0.0119 -0.0274 0.1796 -0.0303 0.2403 34.8613 64.8867 116.4542 10 7.7806 -4.6451 -5.2094 7.778 4.9132 7.3844 -0.3461 -0.7018 -0.4493 0.3108 -0.5726 1.0052 -0.4746 0.0204 -0.613 0.5666 0.2264 -0.2652 0.2728 -0.1976 0.2578 32.7948 78.0801 125.7338 11 6.8725 5.0849 2.6634 4.2898 3.6621 6.4597 -0.7702 1.9645 -1.1721 -1.3764 2.4546 -1.3457 -1.5193 0.2749 -0.9669 0.4143 -0.2736 0.0595 0.5254 -0.1026 0.3021 22.0783 61.9743 40.1814 12 2.7185 2.9171 0.4557 3.6 0.7509 8.3507 -0.5894 -0.1334 0.5169 0.1356 -0.087 1.4216 0.668 -1.6326 0.2723 0.1379 -0.0599 -0.0349 0.2989 0.0045 0.3694 22.0622 62.4913 48.3466 13 1.0646 -1.2298 2.2021 1.6569 -2.9606 5.267 -0.2525 -0.2401 0.7492 0.4324 0.1779 -0.0041 -1.0615 -1.3435 0.1394 0.1948 0.0052 0.0042 0.4026 -0.0325 0.2314 24.3029 63.007 56.0858 14 5.8382 -3.0473 6.4343 4.2323 -0.9601 9.2894 -1.1103 0.2531 1.2015 0.8653 0.2217 0.0474 -0.4017 1.0507 0.8796 0.2075 -0.0551 0.0321 0.3934 -0.0259 0.2999 24.0316 62.8148 65.2454 15 4.6545 3.059 -2.4191 4.0471 -5.213 7.7877 0.3741 0.0086 1.1429 0.5603 -0.1625 0.8514 -1.414 0.1271 0.6266 0.2109 -0 -0.033 0.3006 0.0285 0.2886 26.9773 64.1268 72.5296 16 5.4745 -4.5348 3.9925 5.771 -0.5099 6.7825 -1.4101 0.0506 0.1839 2.3061 0.1421 0.2515 -0.4818 0.2002 0.4821 0.4801 -0.0439 -0.1096 0.2463 0.0238 0.2956 29.5332 63.0857 81.1181 17 5.1715 -0.756 2.7163 2.0192 -1.3825 7.2321 -1.2994 -0.6269 1.0028 0.9099 -0.1677 -0.1501 -1.674 -0.2064 1.2176 0.9327 0.2577 -0.2417 0.1285 -0.0599 0.3446 33.7798 64.0861 90.308 18 5.6104 5.6426 5.3549 6.141 6.5618 8.7599 -1.4149 -0.227 1.3119 -1.8496 0.6428 0.7951 -2.1491 0.5959 0.4238 0.8614 0.1402 -0.0814 0.2198 -0.0003 0.3788 38.8336 63.8093 97.4901 19 2.5567 -1.8913 -0.2128 5.3459 3.4254 2.9083 0.4329 0.3807 0.5218 -1.1161 -0.1822 -0.8067 0.2337 -0.1757 0.1443 0.3133 0.017 0.0482 0.2626 0.0388 0.2209 42.0602 62.2991 107.0357 20 1.8527 1.4581 -1.155 5.722 -0.0566 6.1924 -0.1993 -0.4191 -0.6863 0.2821 -0.296 -0.985 1.5914 -0.1018 0.3983 0.2442 -0.0669 0.0185 0.2007 0.0754 0.2265 44.3678 60.1486 117.7435
Refinement TLS group Show large table (6 x 20) Hide large table ID Refine-ID Refine TLS-ID Selection details Auth asym-ID Auth seq-ID 1 X-RAY DIFFRACTION 1 (chain A and resid 215:220)A215 - 220 2 X-RAY DIFFRACTION 2 (chain A and resid 221:225)A221 - 225 3 X-RAY DIFFRACTION 3 (chain A and resid 226:231)A226 - 231 4 X-RAY DIFFRACTION 4 (chain A and resid 232:236)A232 - 236 5 X-RAY DIFFRACTION 5 (chain A and resid 237:242)A237 - 242 6 X-RAY DIFFRACTION 6 (chain A and resid 243:249)A243 - 249 7 X-RAY DIFFRACTION 7 (chain A and resid 250:256)A250 - 256 8 X-RAY DIFFRACTION 8 (chain A and resid 257:263)A257 - 263 9 X-RAY DIFFRACTION 9 (chain A and resid 264:272)A264 - 272 10 X-RAY DIFFRACTION 10 (chain A and resid 273:277)A273 - 277 11 X-RAY DIFFRACTION 11 (chain B and resid 213:218)B213 - 218 12 X-RAY DIFFRACTION 12 (chain B and resid 219:223)B219 - 223 13 X-RAY DIFFRACTION 13 (chain B and resid 224:229)B224 - 229 14 X-RAY DIFFRACTION 14 (chain B and resid 230:234)B230 - 234 15 X-RAY DIFFRACTION 15 (chain B and
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  SIRAS / Resolution: 2.2001 Å
SIRAS / Resolution: 2.2001 Å  Authors
Authors Citation
Citation Journal: J.Virol. / Year: 2013
Journal: J.Virol. / Year: 2013 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4eij.cif.gz
4eij.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4eij.ent.gz
pdb4eij.ent.gz PDB format
PDB format 4eij.json.gz
4eij.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 4eij_validation.pdf.gz
4eij_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 4eij_full_validation.pdf.gz
4eij_full_validation.pdf.gz 4eij_validation.xml.gz
4eij_validation.xml.gz 4eij_validation.cif.gz
4eij_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/ei/4eij
https://data.pdbj.org/pub/pdb/validation_reports/ei/4eij ftp://data.pdbj.org/pub/pdb/validation_reports/ei/4eij
ftp://data.pdbj.org/pub/pdb/validation_reports/ei/4eij Links
Links Assembly
Assembly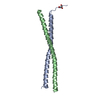


 Components
Components

 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 22-BM / Wavelength: 1 Å
/ Beamline: 22-BM / Wavelength: 1 Å SIRAS
SIRAS Processing
Processing SIRAS / Resolution: 2.2001→26.607 Å / Occupancy max: 1 / Occupancy min: 1 / FOM work R set: 0.8474 / SU ML: 0.26 / σ(F): 0.12 / Phase error: 21.95 / Stereochemistry target values: ML
SIRAS / Resolution: 2.2001→26.607 Å / Occupancy max: 1 / Occupancy min: 1 / FOM work R set: 0.8474 / SU ML: 0.26 / σ(F): 0.12 / Phase error: 21.95 / Stereochemistry target values: ML Movie
Movie Controller
Controller












 PDBj
PDBj


