+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4d7l | ||||||
|---|---|---|---|---|---|---|---|
| Title | Methionine sulfoxide reductase A of Corynebacterium diphtheriae | ||||||
 Components Components | PEPTIDE METHIONINE SULFOXIDE REDUCTASE MSRA | ||||||
 Keywords Keywords | OXIDOREDUCTASE / THIOL DISULFIDE EXCHANGE | ||||||
| Function / homology |  Function and homology information Function and homology information: / peptide-methionine (S)-S-oxide reductase / peptide-methionine (S)-S-oxide reductase activity / protein modification process / cellular response to oxidative stress / cytoplasm Similarity search - Function | ||||||
| Biological species |  CORYNEBACTERIUM DIPHTHERIAE NCTC 13129 (bacteria) CORYNEBACTERIUM DIPHTHERIAE NCTC 13129 (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.895 Å MOLECULAR REPLACEMENT / Resolution: 1.895 Å | ||||||
 Authors Authors | Van Molle, I. / Tossounian, M.A. / Pedre, B. / Wahni, K. / Vertommen, D. / Messens, J. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2015 Journal: J.Biol.Chem. / Year: 2015Title: Corynebacterium Diphtheriae Methionine Sulfoxide Reductase a Exploits a Unique Mycothiol Redox Relay Mechanism. Authors: Tossounian, M. / Pedre, B. / Wahni, K. / Erdogan, H. / Vertommen, D. / Van Molle, I. / Messens, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4d7l.cif.gz 4d7l.cif.gz | 275.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4d7l.ent.gz pdb4d7l.ent.gz | 222.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4d7l.json.gz 4d7l.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4d7l_validation.pdf.gz 4d7l_validation.pdf.gz | 486.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4d7l_full_validation.pdf.gz 4d7l_full_validation.pdf.gz | 490 KB | Display | |
| Data in XML |  4d7l_validation.xml.gz 4d7l_validation.xml.gz | 32.5 KB | Display | |
| Data in CIF |  4d7l_validation.cif.gz 4d7l_validation.cif.gz | 47.7 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d7/4d7l https://data.pdbj.org/pub/pdb/validation_reports/d7/4d7l ftp://data.pdbj.org/pub/pdb/validation_reports/d7/4d7l ftp://data.pdbj.org/pub/pdb/validation_reports/d7/4d7l | HTTPS FTP |
-Related structure data
| Related structure data |  1ff3S S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| 2 | 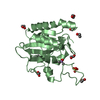
| |||||||||
| 3 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 26145.139 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  CORYNEBACTERIUM DIPHTHERIAE NCTC 13129 (bacteria) CORYNEBACTERIUM DIPHTHERIAE NCTC 13129 (bacteria)Production host:  #2: Chemical | #3: Chemical | ChemComp-SO4 / #4: Chemical | ChemComp-PGE / #5: Water | ChemComp-HOH / | Has protein modification | Y | Sequence details | THE MSRA GENE WAS CLONED IN THE PET28A VECTOR, WITH A N- TERMINAL HIS-TAG AND A THROMBIN CLEAVAGE ...THE MSRA GENE WAS CLONED IN THE PET28A VECTOR, WITH A N- TERMINAL HIS-TAG AND A THROMBIN CLEAVAGE SITE, RESULTING IN THE FOLLOWING ADDITIONAL | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.73 Å3/Da / Density % sol: 54.94 % / Description: NONE |
|---|---|
| Crystal grow | pH: 7 Details: 0.1 M NA CACODYLATE PH 7.0, 0.35 M AMMONIUM SULFATE, 16% PEG8000 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SOLEIL SOLEIL  / Beamline: PROXIMA 1 / Wavelength: 0.97857 / Beamline: PROXIMA 1 / Wavelength: 0.97857 |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Jul 18, 2014 Details: KIRKPATRICK-BAEZ PAIR OF BI-MORPH MIRRORS PLUS CHANNEL CUT CRYOGENICALLY COOLED MONOCHROMATOR CRYSTAL |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97857 Å / Relative weight: 1 |
| Reflection | Resolution: 1.89→50 Å / Num. obs: 67677 / % possible obs: 99.7 % / Observed criterion σ(I): 3 / Redundancy: 8.85 % / Rmerge(I) obs: 0.15 / Net I/σ(I): 11.79 |
| Reflection shell | Resolution: 1.89→2.01 Å / Redundancy: 8.5 % / Rmerge(I) obs: 0.9 / Mean I/σ(I) obs: 2.27 / % possible all: 98.1 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1FF3 Resolution: 1.895→41.291 Å / SU ML: 0.19 / σ(F): 1.35 / Phase error: 18.49 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.895→41.291 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj










