[English] 日本語
 Yorodumi
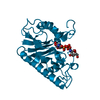
Yorodumi- PDB-4cj8: monoclinic crystal form of Bogt6a E192Q in complex with UDP-GalNA... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4cj8 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | monoclinic crystal form of Bogt6a E192Q in complex with UDP-GalNAc, UDP and GalNAc | |||||||||
 Components Components | GLYCOSYLTRANSFERASE FAMILY 6 | |||||||||
 Keywords Keywords | TRANSFERASE / MONOCLINIC FORM / METAL-INDEPENDENT / HYDROLYSED PRODUCTS | |||||||||
| Function / homology |  Function and homology information Function and homology informationhexosyltransferase activity / carbohydrate metabolic process / nucleotide binding / metal ion binding / membrane Similarity search - Function | |||||||||
| Biological species |  BACTEROIDES OVATUS (bacteria) BACTEROIDES OVATUS (bacteria) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 3.5 Å SAD / Resolution: 3.5 Å | |||||||||
 Authors Authors | Pham, T. / Stinson, B. / Thiyagarajan, N. / Lizotte-Waniewski, M. / Brew, K. / Acharya, K.R. | |||||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2014 Journal: J.Biol.Chem. / Year: 2014Title: Structures of Complexes of a Metal-Independent Glycosyltransferase Gt6 from Bacteroides Ovatus with Udp-Galnac and its Hydrolysis Products Authors: Pham, T.T.K. / Stinson, B. / Thiyagarajan, N. / Lizotte-Waniewski, M. / Brew, K. / Acharya, K.R. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4cj8.cif.gz 4cj8.cif.gz | 797.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4cj8.ent.gz pdb4cj8.ent.gz | 660.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4cj8.json.gz 4cj8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4cj8_validation.pdf.gz 4cj8_validation.pdf.gz | 5.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4cj8_full_validation.pdf.gz 4cj8_full_validation.pdf.gz | 5.2 MB | Display | |
| Data in XML |  4cj8_validation.xml.gz 4cj8_validation.xml.gz | 157.6 KB | Display | |
| Data in CIF |  4cj8_validation.cif.gz 4cj8_validation.cif.gz | 187.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cj/4cj8 https://data.pdbj.org/pub/pdb/validation_reports/cj/4cj8 ftp://data.pdbj.org/pub/pdb/validation_reports/cj/4cj8 ftp://data.pdbj.org/pub/pdb/validation_reports/cj/4cj8 | HTTPS FTP |
-Related structure data
| Related structure data |  4cjbSC  4cjcC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
- Components
Components
-Protein , 1 types, 16 molecules ABCDEFGHIJKLMNOP
| #1: Protein | Mass: 29262.297 Da / Num. of mol.: 16 / Fragment: ACTIVE SITE, RESIDUES 1-246 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)  BACTEROIDES OVATUS (bacteria) / Production host: BACTEROIDES OVATUS (bacteria) / Production host:  References: UniProt: A7LVT2, glycoprotein-fucosylgalactoside alpha-N-acetylgalactosaminyltransferase |
|---|
-Sugars , 2 types, 10 molecules 
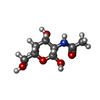

| #4: Sugar | ChemComp-A2G / #8: Sugar | |
|---|
-Non-polymers , 6 types, 30 molecules 



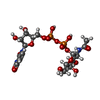






| #2: Chemical | ChemComp-GOL / #3: Chemical | ChemComp-UDP / #5: Chemical | ChemComp-1PE / | #6: Chemical | ChemComp-SO4 / | #7: Chemical | ChemComp-UD2 / #9: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | N |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.5 Å3/Da / Density % sol: 56 % / Description: NONE |
|---|---|
| Crystal grow | pH: 5 / Details: 0.2M LI2SO4 0.1M BIS TRIS, PH 5.5 20% PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 289.15 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.9795 / Beamline: I04 / Wavelength: 0.9795 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Oct 30, 2012 / Details: MIRRORS |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 3.5→87.98 Å / Num. obs: 61949 / % possible obs: 98 % / Observed criterion σ(I): 7.4 / Redundancy: 2.7 % / Rmerge(I) obs: 0.13 / Net I/σ(I): 7.4 |
| Reflection shell | Resolution: 3.5→3.59 Å / Redundancy: 7.4 % / Rmerge(I) obs: 0.7 / Mean I/σ(I) obs: 2.1 / % possible all: 99 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD SADStarting model: PDB ENTRY 4CJB Resolution: 3.5→87.977 Å / σ(F): 1.34 / Phase error: 28.21 / Stereochemistry target values: TWIN_LSQ_F
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.5→87.977 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj