| Entry | Database: PDB / ID: 4bfk
|
|---|
| Title | Superoxide reductase (Neelaredoxin) from Archaeoglobus fulgidus E12Q mutant |
|---|
 Components Components | SUPEROXIDE REDUCTASE |
|---|
 Keywords Keywords | OXIDOREDUCTASE / OXYGEN DETOXIFICATION |
|---|
| Function / homology |  Function and homology information Function and homology information
SOR catalytic domain / Desulfoferrodoxin, ferrous iron-binding domain / Desulfoferrodoxin, ferrous iron-binding domain superfamily / : / Desulfoferrodoxin / Immunoglobulin-like / Sandwich / Mainly BetaSimilarity search - Domain/homology |
|---|
| Biological species |   ARCHAEOGLOBUS FULGIDUS (archaea) ARCHAEOGLOBUS FULGIDUS (archaea) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.103 Å MOLECULAR REPLACEMENT / Resolution: 2.103 Å |
|---|
 Authors Authors | Bandeiras, T.M. / Rodrigues, J.V. / Sousa, C.M. / Barradas, A.R. / Pinho, F.G. / Pinto, A.F. / Teixeira, M. / Matias, P.M. / Romao, C.V. |
|---|
 Citation Citation |  Journal: To be Published Journal: To be Published
Title: Understanding the Role of Key Residues in the Superoxide Reductase Molecular Mechanism, Exploring Archaeoglobus Fulgidus Sor Structure
Authors: Bandeiras, T.M. / Rodrigues, J.V. / Sousa, C.M. / Barradas, A.R. / Pinho, F.G. / Pinto, A.F. / Teixeira, M. / Matias, P.M. / Romao, C.V. |
|---|
| History | | Deposition | Mar 19, 2013 | Deposition site: PDBE / Processing site: PDBE |
|---|
| Revision 1.0 | Mar 26, 2014 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Dec 20, 2023 | Group: Data collection / Database references ...Data collection / Database references / Derived calculations / Other / Refinement description
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / pdbx_database_status / pdbx_initial_refinement_model / pdbx_struct_conn_angle / struct_conn / struct_site
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_sf / _pdbx_struct_conn_angle.ptnr1_auth_comp_id / _pdbx_struct_conn_angle.ptnr1_auth_seq_id / _pdbx_struct_conn_angle.ptnr1_label_atom_id / _pdbx_struct_conn_angle.ptnr1_label_comp_id / _pdbx_struct_conn_angle.ptnr1_label_seq_id / _pdbx_struct_conn_angle.ptnr3_auth_comp_id / _pdbx_struct_conn_angle.ptnr3_auth_seq_id / _pdbx_struct_conn_angle.ptnr3_label_atom_id / _pdbx_struct_conn_angle.ptnr3_label_comp_id / _pdbx_struct_conn_angle.ptnr3_label_seq_id / _pdbx_struct_conn_angle.value / _struct_conn.pdbx_dist_value / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id / _struct_conn.ptnr2_label_seq_id / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 ARCHAEOGLOBUS FULGIDUS (archaea)
ARCHAEOGLOBUS FULGIDUS (archaea) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.103 Å
MOLECULAR REPLACEMENT / Resolution: 2.103 Å  Authors
Authors Citation
Citation Journal: To be Published
Journal: To be Published Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4bfk.cif.gz
4bfk.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4bfk.ent.gz
pdb4bfk.ent.gz PDB format
PDB format 4bfk.json.gz
4bfk.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/bf/4bfk
https://data.pdbj.org/pub/pdb/validation_reports/bf/4bfk ftp://data.pdbj.org/pub/pdb/validation_reports/bf/4bfk
ftp://data.pdbj.org/pub/pdb/validation_reports/bf/4bfk

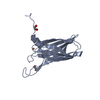


 Links
Links Assembly
Assembly
 Components
Components
 ARCHAEOGLOBUS FULGIDUS (archaea) / Plasmid: PT7-7/NAFE12QA / Production host:
ARCHAEOGLOBUS FULGIDUS (archaea) / Plasmid: PT7-7/NAFE12QA / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ESRF
ESRF  / Beamline: ID23-1 / Wavelength: 0.97925
/ Beamline: ID23-1 / Wavelength: 0.97925  Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller











 PDBj
PDBj

