+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3hsg | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of E. coli HPPK(Y53A) in complex with MgAMPCPP | ||||||
 Components Components | HPPK | ||||||
 Keywords Keywords | TRANSFERASE / alpha beta / ATP-binding / Folate biosynthesis / Kinase / Nucleotide-binding | ||||||
| Function / homology |  Function and homology information Function and homology information2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase / 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase activity / folic acid biosynthetic process / tetrahydrofolate biosynthetic process / kinase activity / magnesium ion binding / ATP binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.14 Å MOLECULAR REPLACEMENT / Resolution: 1.14 Å | ||||||
 Authors Authors | Blaszczyk, J. / Li, Y. / Yan, H. / Ji, X. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: Pterin-binding site mutation Y53A, N55A or F123A and activity of E. coli HPPK Authors: Li, Y. / Blaszczyk, J. / Ji, X. / Yan, H. #1:  Journal: Structure / Year: 2000 Journal: Structure / Year: 2000Title: Catalytic center assembly of HPPK as revealed by the crystal structure of a ternary complex at 1.25 A resolution Authors: Blaszczyk, J. / Shi, G. / Yan, H. / Ji, X. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3hsg.cif.gz 3hsg.cif.gz | 89.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3hsg.ent.gz pdb3hsg.ent.gz | 65.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3hsg.json.gz 3hsg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/hs/3hsg https://data.pdbj.org/pub/pdb/validation_reports/hs/3hsg ftp://data.pdbj.org/pub/pdb/validation_reports/hs/3hsg ftp://data.pdbj.org/pub/pdb/validation_reports/hs/3hsg | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3hsjC  3hszC  3ht0C  1q0nS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 17874.438 Da / Num. of mol.: 1 / Mutation: Y54A Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: P26281, 2-amino-4-hydroxy-6-hydroxymethyldihydropteridine diphosphokinase |
|---|
-Non-polymers , 6 types, 220 molecules 
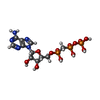









| #2: Chemical | | #3: Chemical | ChemComp-APC / | #4: Chemical | ChemComp-CL / | #5: Chemical | #6: Chemical | #7: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.79 Å3/Da / Density % sol: 31.33 % |
|---|---|
| Crystal grow | Temperature: 292 K / Method: vapor diffusion, hanging drop / pH: 4.6 Details: PEG 4000, Ammonium acetate, Sodium acetate, pH 4.6, VAPOR DIFFUSION, HANGING DROP, temperature 292K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSLS NSLS  / Beamline: X9B / Wavelength: 0.97948 Å / Beamline: X9B / Wavelength: 0.97948 Å |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: Oct 18, 1999 / Details: mirrors |
| Radiation | Monochromator: Silicon 111 / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97948 Å / Relative weight: 1 |
| Reflection | Resolution: 1.14→37.1 Å / Num. all: 46812 / Num. obs: 46812 / % possible obs: 98 % / Observed criterion σ(I): -3 / Redundancy: 5.4 % / Biso Wilson estimate: 6.8 Å2 / Rmerge(I) obs: 0.074 / Χ2: 1.016 / Net I/σ(I): 19.386 |
| Reflection shell | Resolution: 1.14→1.16 Å / Redundancy: 3.4 % / Rmerge(I) obs: 0.733 / Mean I/σ(I) obs: 1.6 / Num. unique all: 2296 / Χ2: 1.028 / % possible all: 97.5 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB entry 1Q0N Resolution: 1.14→32.348 Å / Occupancy max: 1 / Occupancy min: 0.21 / FOM work R set: 0.877 / SU ML: 0.12 Isotropic thermal model: Anisotropic B factors for non-H atoms of full occupancy Cross valid method: THROUGHOUT / σ(F): 1.34 / Stereochemistry target values: ML Details: THE STRUCTURE WAS REFINED FOR A TOTAL OF 40 CYCLES, INCLUDING 3 CYCLES WITH CNS, 15 CYCLES WITH SHELX, AND 22 CYCLES WITH PHENIX
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 78.89 Å2 / ksol: 0.414 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 49.82 Å2 / Biso mean: 14.369 Å2 / Biso min: 3.78 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.14→32.348 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Total num. of bins used: 7
|
 Movie
Movie Controller
Controller











 PDBj
PDBj








