+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3f3a | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of LeuT bound to L-Tryptophan and Sodium | ||||||
 Components Components | Transporter | ||||||
 Keywords Keywords | TRANSPORT PROTEIN / SLC6 / NSS / transmembrane / sodium-coupled / transporter / Symport | ||||||
| Function / homology | Sodium:neurotransmitter symporter / Sodium:neurotransmitter symporter superfamily / Sodium:neurotransmitter symporter family / Sodium:neurotransmitter symporter family profile. / sodium ion transmembrane transport / plasma membrane / TETRADECANE / TRYPTOPHAN / Na(+):neurotransmitter symporter (Snf family) Function and homology information Function and homology information | ||||||
| Biological species |   Aquifex aeolicus (bacteria) Aquifex aeolicus (bacteria) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2 Å MOLECULAR REPLACEMENT / Resolution: 2 Å | ||||||
 Authors Authors | Singh, S.K. / Piscitelli, C.L. / Yamashita, A. / Gouaux, E. | ||||||
 Citation Citation |  Journal: Science / Year: 2008 Journal: Science / Year: 2008Title: A competitive inhibitor traps LeuT in an open-to-out conformation. Authors: Singh, S.K. / Piscitelli, C.L. / Yamashita, A. / Gouaux, E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3f3a.cif.gz 3f3a.cif.gz | 119.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3f3a.ent.gz pdb3f3a.ent.gz | 92 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3f3a.json.gz 3f3a.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  3f3a_validation.pdf.gz 3f3a_validation.pdf.gz | 1.5 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  3f3a_full_validation.pdf.gz 3f3a_full_validation.pdf.gz | 1.5 MB | Display | |
| Data in XML |  3f3a_validation.xml.gz 3f3a_validation.xml.gz | 23 KB | Display | |
| Data in CIF |  3f3a_validation.cif.gz 3f3a_validation.cif.gz | 31.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/f3/3f3a https://data.pdbj.org/pub/pdb/validation_reports/f3/3f3a ftp://data.pdbj.org/pub/pdb/validation_reports/f3/3f3a ftp://data.pdbj.org/pub/pdb/validation_reports/f3/3f3a | HTTPS FTP |
-Related structure data
| Related structure data |  3f3cC  3f3dC  3f3eC  3f48C  3f4iC  3f4jC  2a65S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein / Sugars , 2 types, 8 molecules A

| #1: Protein | Mass: 58077.438 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Aquifex aeolicus (bacteria) / Strain: VF5 / Gene: snf, aq_2077 / Plasmid: pET16b / Production host: Aquifex aeolicus (bacteria) / Strain: VF5 / Gene: snf, aq_2077 / Plasmid: pET16b / Production host:  |
|---|---|
| #3: Sugar | ChemComp-BOG / |
-Non-polymers , 4 types, 108 molecules 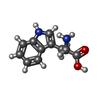






| #2: Chemical | ChemComp-TRP / #4: Chemical | ChemComp-C14 / | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.67 Å3/Da / Density % sol: 53.91 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 7 Details: 0.1M HEPES, 0.4M NaCl, 17-22% PEG-MME 550, pH 7.0, VAPOR DIFFUSION, HANGING DROP, temperature 293K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 8.2.2 / Wavelength: 0.9796 Å / Beamline: 8.2.2 / Wavelength: 0.9796 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Jul 13, 2007 |
| Radiation | Monochromator: Double Crystal Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9796 Å / Relative weight: 1 |
| Reflection | Resolution: 2→50 Å / Num. obs: 41152 / % possible obs: 97.4 % / Redundancy: 2.6 % / Biso Wilson estimate: 31.79 Å2 / Rmerge(I) obs: 0.053 / Net I/σ(I): 11.1 |
| Reflection shell | Resolution: 2→2.07 Å / Redundancy: 2.3 % / Rmerge(I) obs: 0.577 / Mean I/σ(I) obs: 1.1 / Num. unique all: 3834 / % possible all: 90.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB Entry 2A65 Resolution: 2→48.232 Å / SU ML: 0.29 / Cross valid method: THROUGHOUT / σ(F): 1.35 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 50 Å2 / ksol: 0.308 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 41.97 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→48.232 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj







