[English] 日本語
 Yorodumi
Yorodumi- PDB-2zwl: Human factor viia-tissue factor complexed with highly selective p... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2zwl | ||||||
|---|---|---|---|---|---|---|---|
| Title | Human factor viia-tissue factor complexed with highly selective peptide inhibitor | ||||||
 Components Components |
| ||||||
 Keywords Keywords | HYDROLASE/BLOOD CLOTTING / serine protease / alternative splicing / blood coagulation / calcium / cleavage on pair of basic residues / disease mutation / EGF-like domain / gamma-carboxyglutamic acid / glycoprotein / hydrolase / hydroxylation / pharmaceutical / polymorphism / protease / zymogen / lipoprotein / membrane / palmitate / transmembrane / HYDROLASE-BLOOD CLOTTING complex / Secreted | ||||||
| Function / homology |  Function and homology information Function and homology informationactivation of plasma proteins involved in acute inflammatory response / activation of blood coagulation via clotting cascade / coagulation factor VIIa / response to Thyroid stimulating hormone / response to astaxanthin / response to thyrotropin-releasing hormone / response to 2,3,7,8-tetrachlorodibenzodioxine / response to carbon dioxide / serine-type peptidase complex / response to genistein ...activation of plasma proteins involved in acute inflammatory response / activation of blood coagulation via clotting cascade / coagulation factor VIIa / response to Thyroid stimulating hormone / response to astaxanthin / response to thyrotropin-releasing hormone / response to 2,3,7,8-tetrachlorodibenzodioxine / response to carbon dioxide / serine-type peptidase complex / response to genistein / response to vitamin K / positive regulation of leukocyte chemotaxis / positive regulation of platelet-derived growth factor receptor signaling pathway / response to thyroxine / NGF-stimulated transcription / response to cholesterol / cytokine receptor activity / response to growth hormone / positive regulation of positive chemotaxis / Extrinsic Pathway of Fibrin Clot Formation / animal organ regeneration / positive regulation of endothelial cell apoptotic process / positive regulation of blood coagulation / positive regulation of TOR signaling / Transport of gamma-carboxylated protein precursors from the endoplasmic reticulum to the Golgi apparatus / Gamma-carboxylation of protein precursors / Removal of aminoterminal propeptides from gamma-carboxylated proteins / positive regulation of endothelial cell proliferation / BMAL1:CLOCK,NPAS2 activates circadian expression / serine-type peptidase activity / positive regulation of interleukin-8 production / circadian rhythm / protein processing / phospholipid binding / Golgi lumen / response to estrogen / : / extracellular matrix / cytokine-mediated signaling pathway / positive regulation of angiogenesis / blood coagulation / response to estradiol / protease binding / vesicle / response to hypoxia / positive regulation of cell migration / endoplasmic reticulum lumen / signaling receptor binding / serine-type endopeptidase activity / external side of plasma membrane / calcium ion binding / positive regulation of gene expression / cell surface / extracellular space / extracellular region / membrane / plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FOURIER SYNTHESIS / Resolution: 2.2 Å FOURIER SYNTHESIS / Resolution: 2.2 Å | ||||||
 Authors Authors | Kadono, S. / Sakamoto, A. / Kikuchi, Y. / Oh-Eda, M. / Yabuta, N. / Koga, T. / Hattori, K. / Shiraishi, T. / Haramura, M. / Sato, H. ...Kadono, S. / Sakamoto, A. / Kikuchi, Y. / Oh-Eda, M. / Yabuta, N. / Koga, T. / Hattori, K. / Shiraishi, T. / Haramura, M. / Sato, H. / Ohta, M. / Kozono, T. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: Human factor viia-tissue factor complexed with highly selective peptide inhibitor Authors: Kadono, S. / Sakamoto, A. / Kikuchi, Y. / Oh-Eda, M. / Yabuta, N. / Koga, T. / Hattori, K. / Shiraishi, T. / Haramura, M. / Sato, H. / Ohta, M. / Kozono, T. | ||||||
| History |
|
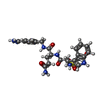
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2zwl.cif.gz 2zwl.cif.gz | 146.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2zwl.ent.gz pdb2zwl.ent.gz | 111.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2zwl.json.gz 2zwl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/zw/2zwl https://data.pdbj.org/pub/pdb/validation_reports/zw/2zwl ftp://data.pdbj.org/pub/pdb/validation_reports/zw/2zwl ftp://data.pdbj.org/pub/pdb/validation_reports/zw/2zwl | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1danS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 3 types, 3 molecules LHT
| #1: Protein | Mass: 17487.076 Da / Num. of mol.: 1 / Fragment: UNP residues 61-212 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: F7 / Cell (production host): CHO / Production host: Homo sapiens (human) / Gene: F7 / Cell (production host): CHO / Production host:  |
|---|---|
| #2: Protein | Mass: 28103.256 Da / Num. of mol.: 1 / Fragment: UNP residues 213-466 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: F7 / Cell (production host): CHO / Production host: Homo sapiens (human) / Gene: F7 / Cell (production host): CHO / Production host:  |
| #3: Protein | Mass: 24697.398 Da / Num. of mol.: 1 / Fragment: UNP residues 33-250 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: F3 / Plasmid: PKK223-3 / Production host: Homo sapiens (human) / Gene: F3 / Plasmid: PKK223-3 / Production host:  |
-Sugars , 2 types, 2 molecules 

| #4: Sugar | ChemComp-BGC / |
|---|---|
| #5: Sugar | ChemComp-FUC / |
-Non-polymers , 3 types, 414 molecules 



| #6: Chemical | ChemComp-CA / #7: Chemical | ChemComp-567 / | #8: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.58 Å3/Da / Density % sol: 52.29 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 5 Details: 8% PEG 5000, 0.1M sodium chloride, 0.005M calcium chloride, 0.1M cacodylate, VAPOR DIFFUSION, HANGING DROP, temperature 298K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU ULTRAX 18 / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU ULTRAX 18 / Wavelength: 1.5418 Å |
| Detector | Type: RIGAKU RAXIS IV / Detector: IMAGE PLATE / Date: Dec 4, 2001 / Details: mirrors |
| Radiation | Monochromator: Confocal Blue Mirror / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.2→50 Å / Num. obs: 37458 / % possible obs: 99.3 % / Observed criterion σ(F): 1 / Observed criterion σ(I): 1 / Redundancy: 3.44 % / Rmerge(I) obs: 0.097 / Net I/σ(I): 15.3 |
| Reflection shell | Resolution: 2.2→2.28 Å / Rmerge(I) obs: 0.345 / Mean I/σ(I) obs: 4.14 / % possible all: 99.5 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: PDB ENTRY 1DAN Resolution: 2.2→30 Å / σ(F): 0 / Stereochemistry target values: Engh & Huber
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.2→30 Å
|
 Movie
Movie Controller
Controller












 PDBj
PDBj














