| Entry | Database: PDB / ID: 2mnw
|
|---|
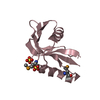
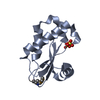
| Title | Solution structure of the P22S mutant of N-terminal CS domain of human Shq1 |
|---|
 Components Components | Protein SHQ1 homolog |
|---|
 Keywords Keywords | PROTEIN BINDING / Dyskerin / Cbf5 / H/ACA |
|---|
| Function / homology |  Function and homology information Function and homology information
box H/ACA snoRNP assembly / telomerase RNA localization to Cajal body / regulation of androgen receptor signaling pathway / Telomere Extension By Telomerase / protein-RNA complex assembly / positive regulation of TORC1 signaling / unfolded protein binding / nucleoplasm / cytosol / cytoplasmSimilarity search - Function Shq1 protein domain / Protein Shq1 / : / SHQ1 protein, Shq1 domain / SHQ1-like, CS domain / CS domain / CS domain profile. / Immunoglobulin-like - #790 / HSP20-like chaperone / Immunoglobulin-like ...Shq1 protein domain / Protein Shq1 / : / SHQ1 protein, Shq1 domain / SHQ1-like, CS domain / CS domain / CS domain profile. / Immunoglobulin-like - #790 / HSP20-like chaperone / Immunoglobulin-like / Sandwich / Mainly BetaSimilarity search - Domain/homology |
|---|
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
|---|
| Method | SOLUTION NMR / simulated annealing |
|---|
| Model details | lowest energy, model1 |
|---|
 Authors Authors | Singh, M. / Wang, Z. / Cascio, D. / Feigon, J. |
|---|
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2015 Journal: J.Mol.Biol. / Year: 2015
Title: Structure and Interactions of the CS Domain of Human H/ACA RNP Assembly Protein Shq1.
Authors: Singh, M. / Wang, Z. / Cascio, D. / Feigon, J. |
|---|
| History | | Deposition | Apr 16, 2014 | Deposition site: BMRB / Processing site: RCSB |
|---|
| Revision 1.0 | Jan 14, 2015 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Feb 25, 2015 | Group: Database references |
|---|
| Revision 1.2 | Jun 14, 2023 | Group: Data collection / Database references / Other
Category: database_2 / pdbx_database_status ...database_2 / pdbx_database_status / pdbx_nmr_software / pdbx_nmr_spectrometer / struct_ref_seq_dif
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_nmr_data / _pdbx_nmr_software.name / _pdbx_nmr_spectrometer.model / _struct_ref_seq_dif.details |
|---|
| Revision 1.3 | May 15, 2024 | Group: Data collection / Database references / Category: chem_comp_atom / chem_comp_bond / database_2 / Item: _database_2.pdbx_DOI |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Homo sapiens (human)
Homo sapiens (human) Authors
Authors Citation
Citation Journal: J.Mol.Biol. / Year: 2015
Journal: J.Mol.Biol. / Year: 2015 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2mnw.cif.gz
2mnw.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2mnw.ent.gz
pdb2mnw.ent.gz PDB format
PDB format 2mnw.json.gz
2mnw.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 2mnw_validation.pdf.gz
2mnw_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 2mnw_full_validation.pdf.gz
2mnw_full_validation.pdf.gz 2mnw_validation.xml.gz
2mnw_validation.xml.gz 2mnw_validation.cif.gz
2mnw_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/mn/2mnw
https://data.pdbj.org/pub/pdb/validation_reports/mn/2mnw ftp://data.pdbj.org/pub/pdb/validation_reports/mn/2mnw
ftp://data.pdbj.org/pub/pdb/validation_reports/mn/2mnw Links
Links Assembly
Assembly
 Components
Components Homo sapiens (human) / Gene: SHQ1 / Plasmid: pET41 Ek/LIC / Production host:
Homo sapiens (human) / Gene: SHQ1 / Plasmid: pET41 Ek/LIC / Production host: 
 Sample preparation
Sample preparation Processing
Processing Movie
Movie Controller
Controller












 PDBj
PDBj


 HSQC
HSQC